Abstract
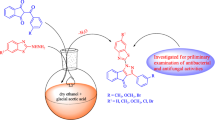
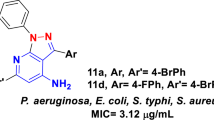
Some new 4H-pyrrolo[1,2-a]benzimidazoles(2a–2f) have been synthesized. The structures of these compounds were confirmed by IR, 1H NMR, mass spectroscopy and elemental analysis. Compound 2a was further confirmed by X-ray diffraction. The in vitro antimicrobial activities of these compounds were determined against some gram-positive bacteria, gram-negative bacteria and fungi and their drug-resistant isolates in comparison with standard drugs. Antimicrobial results indicate that compounds 2c, 2d and 2e show moderately active antibacterial properties, their minimum inhibitory concentrations are from 12.5 μg/mL to 125 μg/mL. In the series, the most active compound against C. albicans is compound 2f with an MIC value of 31.25 μg/mL.
Similar content being viewed by others
References
Zhou Y., Wang D. M., Zhao X. G., Zhou H. W., Chen C. H., Dang G. D., Chem. J. Chinese Universities, 2013, 34(10), 2427
Ruiz V. R., Avelino C., Sabater M. J., Tetrahedron, 2010, 66(3), 730
Kumar D., Jacob M. R., Reynoldsa M. B., Kerwin S. M., Bioorg. Med. Chem., 2002, 10(12), 3997
Demirayak S., Kayagil I., Yurttaset L., Eur. J. Med. Chem., 2011, 46(1), 411
Refaat M. H., Eur. J. Med. Chem., 2010, 45(7), 2949
Hranjec M., Kralj M., Piantanida I., Sedic M., Sÿuman L. D., Karminski-Zamola G., J. Med. Chem., 2007, 50(23), 5696
Piskin A. K., Ates-Alagoz Z., Turk. J. Biochem., 2009, 34, 39
Zhou R., Skibo E. B., J. Med. Chem., 1996, 39(21), 4321
Craigo W. A., le Sueur B. W., Skibo E. B., J. Med. Chem., 1999, 42(17), 3324
Suleman A., Skibo E. B., J. Med. Chem., 2002, 45(6), 1211
Xing C. G., Wu P., Skibo E. B., J. Med. Chem., 2000, 43(3), 457
Schulz W. G., Islam I., Skibo E. B., J. Med. Chem., 1995, 38(1), 109
Nehal M., Elwan N. M., Tetrahedron, 2004, 60(5), 1161
Awadallah M., Seppeltb A., Shorafa K. H., Tetrahedron, 2006, 62(33), 7744
Despina A., Carnpi M. E., Fallon G. D., Aust. J. Chem., 1993, 46, 1623
Ogura H., Kikuchi K., J. Org. Chem., 1972, 37(17), 2679
Matsuda Y., Heterocycle, 1992, 33, 295
Watanabe H., Tsuge O., J. Org. Chem., 1989, 54(2), 420
Wang B. X., Hu J. X., Zhang X. C., Hu. H. W., J. Heterocycl. Chem., 2000, 37(6), 1533
Shen Z. Y., Han Q. R., Wang B. X., Shen J., J. Function. Mater., 2008, 4, 536
Wei X., Hu Y., Li T., J. Chem. Soc., Perkin Trans I., 1993, 2487
Wang B. X., Li J., Jiang X., Hu H. W., J. Chem. Soc., Perkin Trans I., 1999, 1571
Rohini R., Reddy P. M., Shanker K., Huc A., Ravinder V., Eur. J. Med. Chem., 2010, 45(3), 1200
Reddy P. M., Ho Y. P., Shanker K., Rohini R., Ravinder V., Eur. J. Med. Chem., 2009, 44(6), 2621
Andrews J., Antimicrob Agents Chem., 2010, 54, 5
Oludotun A. P., Edet E. U., Santhosh M. S., Eur. J. Med. Chem., 2008, 43(5), 1095
Kotretsou S., Mingeot-Leclercq M. P., Constantinou-Kokotou V., Brasseur R., Georgiadis M. P., Tulkens P. M., J. Med. Chem., 1995, 38(23), 4710
Chaudhari B. R., Rindhe S. S., J. Serb. Chem. Soc., 2010, 76, 1199
Al-Tel T. H., Al-Qawasmeh R. A., Zaarour R., Eur. J. Med. Chem., 2011, 46(5), 1874
Ansari K. F., Lal C., Eur. J. Med. Chem., 2009, 44(10), 4028
Ansari K. F., Lal C., Eur. J. Med. Chem., 2009, 44(5), 2294
Alper-Hayta S., Arisoy M., Temiz-Arpaci Ö., Kaynak F., Eur. J. Med. Chem., 2008, 43(11), 2568
Ghali R. M., Hashim R., Alshahateet S. F., Mehdi S. H., Sulaiman O., Murugaiyah V., Aruldass C. A., J. Mole. Struct., 2011, 1005(1), 152
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Natural Science Foundation of Jiangsu Education Committee, China(No.12KJD15008) and the National Natural Science Foundation of China(No.21202101).
Rights and permissions
About this article
Cite this article
Jiang, Y., Han, Q., Shen, R. et al. Synthesis and antimicrobial activity of some new 4H-pyrrolo[1,2-a]benzimidazoles. Chem. Res. Chin. Univ. 30, 755–758 (2014). https://doi.org/10.1007/s40242-014-4147-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-014-4147-2