Abstract
The number of people surviving sudden cardiac arrest (SCA) is increasing. Clinical and population-based sciences are improving lay chest compression rates and use of automated electronic defibrillators, which will continue to have an impact on future SCA victims, and subsequently, the number of survivors requiring acute and rehabilitation care. A large research and best clinical practice guideline gap remains, however. The continuum of care for rehabilitation and recovery after SCA is often fragmented and underdeveloped, particularly for those requiring specialized services due anoxic brain injury resulting from the SCA. While some elements of rehabilitation care are similar to that provided to other brain injury survivors (e.g., traumatic brain injury), the unique pathology associated with a global insult like anoxic brain injury may require modifications to standard therapies. The purpose of this review is to describe the clinical evidence and best practices for optimizing recovery after SCA. It will further introduce experiential and research-derived concepts for neurorehabilitation care for the subpopulation of SCA survivors with concurrent anoxic brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background/Epidemiology
Sudden cardiac arrest (SCA) is estimated to affect 365,500 people outside of the hospital each year in the USA [1]. Etiology varies by age. Respiratory arrest is more common in neonates and children; drug overdoses and arrhythmias are common in adolescents or young adults; and drug overdoses, coronary artery disease, and arrhythmias are most common in adults [2]. Recent targeted efforts to improve rates of bystander initiated cardiopulmonary resuscitation (CPR) include mass public training in compression-only CPR, 911 dispatch-assisted telephone instruction, state legislation to make CPR training mandatory in schools, and airport kiosk simulators that teach and promote chest compression quality [3].
Over the past 15 years, survival from SCA has improved in multiple countries, with large database estimates ranging from 6 to 8% in 2006 to 10–12% in 2014, regardless of initial arrest rhythm or age [4, 5]. About two thirds of deaths occur prior to admission to the intensive care unit because CPR fails to reverse cardiac arrest. Among individuals admitted to the hospital after CPR, about 70% die in the hospital as a consequence of brain injury [6]. Among survivors discharged from the hospital, many achieve “good” function, as typically measured and reported by Cerebral Performance Category or modified Rankin scale [7, 8]. Despite good function on these broad scales, cognitive or physical impairments, affective disturbances, and decreased return to premorbid activities commonly occur among SCA survivors [9, 10].
The optimum outcome measurement tools for SCA, and the timing of their measurement, remain unknown [11]. In contrast to other disease states, such as stroke, cancer, and solid-organ transplant, core outcome sets and patient-centered values after cardiac arrest are only recently being assessed and prioritized for their relevance to SCA [12, 13]. However, SCA survivors comprise an increasing percentage of individuals treated in both inpatient and outpatient rehabilitation settings. As survival rates continue to increase, the importance of addressing SCA survivor symptoms and sequelae, including neurological symptoms/sequelae associated with anoxic brain injury, will continue to rise.
Recognizing that survival after SCA is more complex than “alive versus dead,” it is important to note that there are no standard guidelines or recommendations for assessment and treatment of SCA survivors prior to or after discharge from the acute care hospital. This research gap contributes, in part, to our lack of understanding regarding how this subset of patients, and their families, experience life after discharge from the hospital. Additionally, we lack comprehensive understanding of what long-term challenges they face, which may impact quality of life for months to years or more [14]. SCA survivors may have similar needs to other patients requiring intensive care or to those who sustained a traumatic or other acquired brain injury (e.g., hypoxic brain injury) or who did not suffer cardiac arrest but required an automatic implantable cardioverter defibrillator (ICD); however, differences in functional needs between groups have not been studied. This review highlights domains important to consider in the rehabilitation of SCA survivors, including their families and communities, and identifies needed areas of rehabilitation research for this population.
Initial Assessments and Prognostication
Patients suffering cardiac arrest who wake up immediately after resuscitation fare well, although the amount of time required to cause permanent ischemia-reperfusion injury [15] to the body, especially the brain, may be different among individuals. A “short” period of pulselessness, such as 1–5 min, may produce subtle to no hypoxic brain injury effects in some people, depending on the cognitive assessments or outcome measures employed. Anecdotally, patients can be neurologically devastated after only a few minutes of arrest and survived fully “neurologically intact” after prolonged periods (>20–30 min) of arrest [16, 17]. The performance characteristics of imaging and biomarker tests limit clinical abilities to prognosticate the outcome for a comatose survivor of cardiac arrest [18], which is further confused because clinicians vary widely in the time they may wait for awakening before withdrawal of care [19]. While several US case series [20,21,22,23] have suggested that delayed awakening is common, variations in medical provider decision-making and family counseling at the time of providing critical, life-sustaining therapies confound studies using combined data sets to evaluate who survives cardiac arrest.
Medical Issues and Considerations
Survivors of cardiac arrest in acute care may have new or ongoing, concurrent medical needs, managed by various subspecialists. New medical concerns may range from the addition of daily medications (e.g., antihypertensives, antiepileptics, and mood stabilizers) and the need for an ICD to new onset renal or respiratory failure, potentially requiring long-term hemodialysis or ventilator dependence and tracheostomy care. The previously healthy patient may have a difficult time accepting the need for lifestyle changes and chronic medical care, while patients with prior medical conditions may need additional support integrating new medical specialists with established care providers. Broad education across the patient’s system of care is needed to facilitate patient-centered comprehensive care.
The post-cardiac arrest patient shares many problems with other graduates of intensive care. For example, long-term mechanical ventilation may have resulted in new tracheostomy, vocal cord dysfunction, dysphagia, or other pulmonary compromise. Other procedures, such as central venous and arterial lines, dialysis catheters, chest tubes, or drains, may leave residual pain or healing wounds. Coma and critical illness may contribute to significant weight loss, deconditioning, and even myopathy. Specific to survivors of CPR, multiple rib fractures can result in chest wall pain and issues with pulmonary toilet. Anecdotally, patients who had CPR report pain most commonly at the sternal-costal junction, which is reminiscent of the post-cardiac surgery patient who has sternotomy pain. Similar therapeutic approaches may benefit these complaints (cough pillow, incentive spirometry, systemic, or local non-steroidal analgesic medications).
Depending on the etiology of arrest, both pediatric and adult survivors may face long-term anxiety regarding secondary prevention of recurrent events. Patients may be discharged with a temporary life vest until it is determined that an ICD is needed. Significant research has been done with patients who require an ICD and their families, and findings demonstrate the fear associated with potentially being shocked by a new device and the post-traumatic stress resulting from a device firing, either appropriately or inappropriately [24, 25]. Research further suggests that patients may alter behavior, avoid physical exertion, and have decreased quality of life as a result [26, 27]. These issues require consideration when formulating an approach to rehabilitation and recovery; they have the potential to influence relationships with family, friends, and coworkers, thereby affecting the patient’s ability to function in society.
Neurological Injury and Sequelae
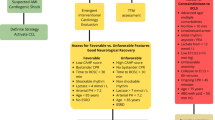
SCA survivors have the potential for disorders of consciousness (DOC) as well as various motor, functional, cognitive, behavioral, and emotional difficulties (see Table 1). While many of these problems overlap in presentation with other acquired forms of brain injury, the underlying pathology can be quite different. As such, some unique approaches to treatment are emerging as we gain more experience evaluating and treating symptoms and sequelae that are present among these individuals. In general, recovery from significant anoxic brain injury typically is considered to be more limited than recovery from traumatic brain injury (TBI) [28,29,30]. However, SCA survivors with brain injury are an emerging subgroup of anoxic brain injury survivors, and more research is needed to define the natural history of their recovery.
While typically less well studied compared to TBI, individuals with anoxic brain injury (from multiple etiologies, including SCA) are often at risk for many of the same complications (e.g., mental health, cognitive deficits, seizures, spasticity) observed with TBI and other types of acquired brain injury [14]. The advent of intensive care treatment programs that include targeted temperature management has resulted in longer durations of support and more intensive monitoring for comatose patients after cardiac arrest [31]. Some long-term survivors have experienced post-cardiac arrest seizures, which were long thought to be untreatable, and require titration or weaning of anticonvulsant medications [32].
The high prevalence of movement disorders in the anoxic brain injury population suggests unique differences in etiology and pathological vulnerabilities compared to TBI or other acquired brain injuries. Our clinical experience and that of others suggest that myoclonus, tremor, chorea, and other less well-defined motor restlessness can occur frequently after anoxic brain injury, including that arising from SCA [33]. Ischemic vulnerability, leading to disrupted basal ganglia and cerebellar circuitry, may contribute to these findings [34, 35].
Models of Comprehensive Post-Cardiac Arrest Care
Some acute health care centers have developed a niche in post-cardiac arrest care, including protocolized rehabilitation assessment and treatment plans. At these institutions, a special post-cardiac arrest team, including emergency medicine, critical care, cardiology, and neurology providers, coordinates acute inpatient medical care and subspecialty consultations. As patients on-service stabilize, multidisciplinary care pathways incorporate specialists from physical medicine and rehabilitation, speech language pathology, and physical and occupational therapy. Early considerations for discharge planning prioritize rehabilitation services. Post-discharge follow-up with patients and families is often a routine extension of the team’s acute care pathway [36]. However, research is needed to determine if/how these multidisciplinary treatment and management algorithms affect health care utilization and functional recovery over the long term.
Rehabilitation physicians that are a part of the post-cardiac arrest team are tasked with considering the potential for participation and medical readiness for patients after SCA. Patients can have a wide range of presentations and hospital courses, making prognosis a challenge. Further, patients can have a wide range of functional deficits despite minimal radiographic findings. For individuals with severe neurological injury, evoked potentials can be informative for identifying patients with vanishingly small chance of recovering cortical functions in addition to other clinical findings [37, 38]. Initial acute care pharmacological interventions may be aimed at improving arousal and participation (see Table 1). Acute care rehabilitation physicians need to also consider each individual’s comorbid conditions, and the influence of such conditions on neuropharmacology selection and exercise tolerance, in anticipation of whether a proposed level of post-acute care rehabilitation is appropriate. For those who need post-acute inpatient care, the need for ICD placement, as well as tracheostomy and feeding tube placement, should be established as a condition for transition to the next level of care.
Acute care discharge planning for SCA survivors is also an opportune time to teach or review the principles of basic life support with the patient and family. Some hospitals have instituted programs to teach chest compressions (CPR) and automated external defibrillator (AED) use, routinely, prior to patient discharge. This is especially important since families are often the persons likely to witness the patient have another cardiac arrest. Epidemiological studies have found that family bystanders often are less likely to initiate CPR [39, 40]. The same opportunity for reviewing basic life support is available at some institutions for parents of newborns prior to discharge [41], arming parents with skills to respond to the child who stops breathing, is choking, or who drowns. Simple interventions targeted at individuals at the point of health system transitions have the potential to impact communities and generations, cultivating a culture of action and preparedness for cardiopulmonary emergencies.
Most hospitals with low numbers of survivors, or few resources for a post-cardiac arrest team approach, may need to utilize other existing infrastructures to deliver similar care. In this case, the first step is recognizing the SCA population and developing a system to integrate them into existing pathways prior to discharge. Existing pathways may include TBI and stroke programs, given similarities with the anoxic brain injury associated with the cardiac arrest. Also, congestive heart failure and cardiac device (ICD) education programs have some overlap in treatment goals, since many SCA patients require heart function and ICD monitoring after discharge. Further, there is an emerging awareness of post-intensive care unit syndromes, wherein there are overlapping concerns for post-ICU critical illness myopathy, sleep disturbances, and cognitive impairment. Pilot ICU follow-up clinics have been established to address these areas of concern and the post-ICU follow-up fragmentation; however, evidence for resultant-improved, patient-centered outcomes remains scarce [42]. Outside of electronic notifications, widespread education and nurse or physician champions may be needed to ensure that SCA patients are referred to existing pathways.
Hospital-based systems targeting care toward the SCA survivor population will be imperfect, especially when patients “look well” and are discharged home quickly. Primary care providers, including pediatricians, require education and awareness for common challenges after surviving cardiac arrest. These providers may have the prime opportunity to screen patients for rehabilitation needs and/or refer them to a rehabilitation specialist for further care. The sheer number of potential referring providers is huge, however, and individuals in need of rehabilitation care may still be missed. Novel outpatient care algorithms to capture patients include improved awareness and screening among cardiac rehabilitation programs and device specialists and improved coordination of care between hospital and outpatient providers. These algorithms are similar to oncology survivorship plans, which originate with the oncology team and guide primary care providers regarding long-term treatment sequelae, screening recommendations, and the need for potential emotional and mental health support [43,44,45,46].
Finally, SCA survivors who are discharged to skilled nursing facilities (SNFs) or long-term acute care (LTAC) facilities also represent a population with potential missed opportunities for rehabilitation care. The number of SCA survivors in this group is estimated at 30–35% of survivors at hospital discharge [47]. Patients may not receive rehabilitative services for various reasons, including the perception that significant improvement in physical or cognitive functioning is unlikely. Without sufficient assessments for recovery, services may not be offered to prevent common complications after SCA (e.g., contractures, decubiti) and to minimize caregiver burden. As rehabilitative research and care progresses for this population, there may be new opportunities for patients to progress and recover to a greater degree than is currently taking place. Thus, as an extension of the hospital system, these facilities have the opportunity to continue systems for assessing and treating SCA patients for rehabilitation needs.
Rehabilitation Care Considerations
Acute rehabilitation providers can provide valuable input differentiating disorders of consciousness (DOC) from locked-in syndrome among those with severe neurological insult after SCA, as well as help identifying conditions (e.g., critical illness neuropathy/myopathy) that can confound prognostic examinations and impact rehabilitation outcomes. Among those with DOC, metrics like the JFK Coma Recovery Scale can provide quantitative assessment documenting temporal changes in level of arousal and interaction with the environment [48, 49]. This information, in conjunction with electrophysiology testing, electroencephalography, and physical exam findings, can provide important prognostic information [50] helpful in determining transitions of care and potential to benefit from rehabilitation therapies and interventions. Increasingly, DOC specialty rehabilitation care programs treat SCA survivors aggressively to improve arousal and participation as well as work to prevent secondary complications arising from the anoxic insult associated with SCA (see Table 1). Although recovery from anoxic brain injury is thought generally to be more limited when compared to individuals with TBI [28] recent work suggests that meaningful recovery can occur among individuals with DOC [28, 51,52,53]. More work is needed to quantify the functional impact that can be made among individuals with DOC due to SCA, as our clinical experience suggests that some do emerge from their vegetative or minimally conscious state.
Given some similarities in presentation, rehabilitation management of the conditions and complications associated with anoxic brain injury can often overlap with management strategies used for treating individuals with other types of acquired TBI. For example, approaches to spasticity management with TBI versus anoxic brain injury due to cardiac arrest are very similar. However, the pathology associated with a global, anoxic brain injury does differ significantly from the regional (e.g., inferior frontal lobes) and mechanical injury mechanisms (e.g., axonal stretch) that accompany TBI. Selectively, vulnerable structures, including the basal ganglia, cerebellum, and hippocampus, sustain significant damage after anoxia that results in cognitive and behavioral deficits, as well as significant movement disorders such as myoclonus, chorea, tremors, and motor restlessness (e.g., akathisia). Recent unpublished data in a rodent model of experimental cardiac arrest suggest that a hyperdopaminergic environment in the striatum in the post-acute period, 2 weeks after insult, worsens with acute dosing of the dopamine agonist methylphenidate [54]. These findings may be relevant to unique clinical pathology associated with anoxic insult. Although further study is needed, potential underlying mechanisms for post-arrest hyperdopaminergia may be due to selective vulnerability of GABAergic medium spiny neurons and/or aspiny cholinergic neurons [55,56,57], pathology that can result in both impaired arousal as well as movement disorders [57].
Dopaminergic agents are a mainstay for improving arousal and cognition among those with TBI. However, in some individuals with anoxia, particularly those with baseline movement disorders, anecdotal evidence suggests that dopaminergic agonists can worsen motor symptoms as well as cognitive deficits and reduce level of arousal. Our experience with this population also suggests that cholinergic drugs like donepezil, as well as the non-benzodiazepine GABA-A receptor agonist zolpidem, can be beneficial in raising the level of arousal as well as reducing severity of movement disorders [34]. Others suggest that after anoxia, zolpidem can increase metabolism in the dorsolateral prefrontal cortex and mesiofrontal cortices, as measured with FDG-PET, and this increase accompanies emergence from DOC [58], presumably by reestablishing GABAergic input from the globus pallidus and increasing thalamocortical activity. Interestingly, studies have shown that some individuals with severe motor symptoms due to Parkinson’s disease can have a significant improvement with symptoms (often without sedating side effects) from zolpidem treatment, presumably through direct drug effects on the globus pallidus [59]. Others suggest anecdotal evidence that cholinergic agonism can improve delirium and level of arousal among individuals with basal ganglia and basal forebrain infarct [60], in the setting of opioid-induced sedation [61] and anticholinergic toxicity [62], and among individuals with poor response to atypical antipsychotics [63,64,65]. Each of these scenarios for cholinergic therapy can be relevant to consider when managing individuals with post-SCA arousal or delirium, especially considering the frequency with which SCA is precipitated by opioid abuse and the frequency with which atypical antipsychotics are used for delirium/agitation management across multiple diagnostic groups.
For those with DOC surviving a severe SCA insult, non-pharmacological sensory stimulation approaches are also used during inpatient rehabilitation to increase level of arousal and participation [66, 67]. In addition, family education, range of motion, positioning, and adaptive equipment evaluations can also prevent complications, support the recovery process, and increase the chances for community-based discharge for these individuals.
In addition to the potential concurrent beneficial effects of cholinergic and non-benzodiazepine GABAergic medications on arousal noted above, our experience is that dopaminergic agents can exacerbate motor symptoms such as chorea, myoclonus, and development of tics. These findings may be consistent with our experimental work showing increased striatal dopaminergia with dopamine agonist challenge. Levetiracetam is a mainstay for treatment of myoclonus after SCA, along with other treatments such as valproate and benzodiazepines, particularly clonazepam [68]. Also, non-pharmacological considerations are a common component to the rehabilitation treatment plan, including carefully conceived mobility plans to limit fall risk. Consideration for adaptive equipment (e.g., weighted utensils) for activities of daily living and experimentation with multisensory feedback and utilization of closed kinetic chain exercise programs may also be beneficial.
While the literature is sparse on long-term emotional and behavioral functioning after SCA, data suggests that behavioral and mental health problems are common during recovery from SCA [69,70,71]. The degree of anoxic brain injury accompanying SCA survival likely influences the risk and symptom profiles observed after injury. Our experience suggests that those with significant anoxic brain injury may experience worsening behavioral symptoms and agitation with dopamine agonists. In addition, individuals with history of drug overdose as an etiologic factor in their presentation may have different risk and symptom profiles. In this latter case, premorbid mental health history and a clear history of substance abuse could be significant factors in effectively addressing behavioral symptoms and agitation after SCA.
For some, there may be anxiety related to cardiac risk for subsequent SCA. Others may experience depression as they struggle to adjust to their level and type of disability. Impaired insight and also impaired capacity for lifestyle change may hinder recovery and quality of life [14], which may, in turn, affect healthy lifestyle choices that affect risk for a subsequent event. While it is unknown if/how antidepressants affect depression after anoxic brain injury, available literature links some types of antidepressants to increased risk for SCA [72]. Thus, care and caution should be taken when prescribing these medications. Importantly, non-pharmacological treatment modalities, particularly cognitive-behavioral therapy, may be quite effective in addressing symptoms related to ICDs [73, 74]. Other approaches, including emotional support groups, may also have therapeutic value in elevating quality of life [75].
Those with anoxic brain injury due to cardiac arrest can be at risk for visual problems, including cortical blindness, perhaps related to high metabolic activity of the occipital cortex and a more tenuous posterior circulation [76]. Hypoxia and hypotension associated with SCA can also lead to ischemic optic neuropathy [77], disturbances in binocular vision [78], and other visuo-perceptual deficits. In cases where vision deficits are suspected, both quantitative (e.g., visual evoked potentials) and functional (e.g., occupational therapy assessment) may be useful to characterize the nature and severity of the problem. Rehabilitation approaches may include low-vision adaptations, orthoptics, integrative sensorimotor vision therapy, and neurovisual processing therapy to reduce the impact of vision deficits and improve visual-perceptual skills.
Community Transitions and Support
Support groups and counseling for patients diagnosed with various medical conditions such as cancer, diabetes, alcoholism, and congenital heart disease, to name a few, are fairly well established. Some programs enable established patients to visit those newly diagnosed in the hospital in order to make connections early. In the USA, the number of adult support groups for SCA survivors and family members is growing, often made possible via the Internet. A few examples include Sudden Cardiac Arrest Association (SCAA) [79], which has numerous state and regional chapters; Sudden Cardiac Arrest Foundation (SCAF) [80]; Take Heart America [81]; HeartRescue Project [82]; and SaveMiHeart [83], which are collaborations of health care system providers and which also provide significant patient support. Parent Heart Watch [84], which focuses on heart screening and prevention of SCA in children, is another example. There are other groups with varying reach and scope, but all serve a need for collaborative support and mission. How, when, and why people join these groups is largely unknown, although discussing experiences and sharing feelings with others is a common motivation [85].
Referral to support groups and counseling after SCA requires health care provider awareness about the existence of such groups, as well as an understanding of the potential benefits to patients and family members. This is yet another resource that may not be available in health care systems or regions with low numbers of survivors. Some patients who are high functioning enough to navigate the Internet and social media may find groups with an active presence. Unlike face-to-face meetings, social media allow people to organize and meet, without constraints of time or space. The fact that several virtual groups exist suggests that there is a need for support that is potentially unfilled by conventional health systems.
Depending on arrest etiology, patient families may require screening for inheritable causes of SCA, such as Brugada or long QT syndrome [86]. The recommendation for screening can often increase anxiety within the entire family unit. Resources for educating and counseling entire families or units of caregivers on arrest etiology and risks to current or future offspring are needed and have the potential to impact generations. Unfortunately, genetic screening may not be well covered by health insurance plans, adding financial stressors to an already difficult situation. As more data are available regarding how and when screening for inheritable causes of SCA are optimal, patients and payers will need to share the burden of prioritizing prevention, similar to that already being done for other medical conditions (e.g., newborn screenings, cancer genetics, cardiovascular risk reduction).
Conclusion
Surviving cardiac arrest is no longer a rare event. There is a growing need across the medical system for awareness of the potential challenges patients and their families face after discharge from the hospital. Rehabilitation assessments and treatments are central to successful cardiac arrest survivorship. Further research on systems of care after cardiac arrest, as well as understanding and managing the medical and neurological sequelae accompanying cardiac arrest, will improve outcomes for this growing population.
References
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, DK MG, Mohler 3rd ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee: Stroke Statistics Subcommittee, Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360.
Meyer L, Stubbs B, Fahrenbruch C, Maeda C, Harmon K, Eisenberg M, Drezner J. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age. Circulation. 2012;126:1363–72.
Bobrow BJ, Spaite DW, Vadeboncoeur TF, Hu C, Mullins T, Tormala W, Dameff C, Gallagher J, Smith G, Panczyk M. Implementation of a regional telephone cardiopulmonary resuscitation program and outcomes after out-of-hospital cardiac arrest. JAMA Cardiol. 2016;1(3):294–302.
Daya MR, Schmicker RH, Zive DM, Rea TD, Nichol G, Buick JE, Brooks S, Christenson J, MacPhee R, Craig A, Rittenberger JC, Davis DP, May S, Wigginton J, Wang H. Resuscitation outcomes consortium investigators. Out-of-hospital cardiac arrest survival improving over time: results from the resuscitation outcomes consortium (ROC). Resuscitation. 2015 Jun;91:108–15.
Vellano K, Crouch A, Rajdev M, McNally B. Cardiac Arrest Registry to Enhance Survival (CARES) report on the public health burden of out-of-hospital cardiac arrest. 2015. [June 30, 2015]. (Paper commissioned by the Committee on Treatment of Cardiac Arrest: Current Status and Future Directions). http://www.iom.edu/~/media/Files/Report%20Files/2015/CARES.pdf.
Callaway CW, Schmicker RH, Brown SP, Albrich JM, Andrusiek DL, Aufderheide TP, Christenson J, Daya MR, Falconer D, Husa RD, Idris AH, Ornato JP, Rac VE, Rea TD, Rittenberger JC, Sears G, Stiell IG. ROC investigators. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85(5):657–63.
Brain Resuscitation Clinical Trial II Study Group. A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med. 1991;324(18):1225–31.
Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2:200–15.
Raina KD, Rittenberger JC, Holm MB, Callaway CW. Functional outcomes: one year after a cardiac arrest. Biomed Res Int. 2015;2015:283608.
Stiell IG, Nesbitt LP, Nichol G, Maloney J, Dreyer J, Beaudoin T, Blackburn J, Wells GA, OPALS Study Group. Comparison of the cerebral performance category score and the health utilities index for survivors of cardiac arrest. Ann Emerg Med. 2009;53(2):241–8.
Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, Nadkarni VM, Abella BS, Adrie C, Berg RA, Merchant RM, O’Connor RE, Meltzer DO, Holm MB, Longstreth WT, Halperin HR. American Heart Association emergency cardiovascular care committee.; council on cardiopulmonary, critical care, perioperative and resuscitation. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124(19):2158–77.
Whitehead L, Perkins GD, Clarey A, Haywood KL. A systematic review of the outcomes reported in cardiac arrest clinical trials: the need for a core outcome set. Resuscitation. 2015 Mar;88:150–7.
Trzeciak S, Jones AE, Kilgannon JH, Fuller BM, Roberts BW, Parrillo JE, Farrar JT. Outcome measures utilized in clinical trials of interventions for post-cardiac arrest syndrome: a systematic review. Resuscitation. 2009;80(6):617–23.
Boyce-van der Wal LW, Volker WG, Vliet Vlieland TP, van den Heuvel DM, van Exel HJ, Goossens PH. Cognitive problems in patients in a cardiac rehabilitation program after an out-of-hospital cardiac arrest. Resuscitation. 2015;93:63–8.
Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth Jr WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand council on resuscitation, European resuscitation council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, resuscitation Council of Asia, and the resuscitation Council of Southern Africa); the American Heart Association emergency cardiovascular care committee; the council on cardiovascular surgery and anesthesia; the council on cardiopulmonary, perioperative, and critical care; the council on clinical cardiology; and the stroke council. Circulation. 2008;118(23):2452–83.
He F, Xu P, Wei ZH, Zhang J, Wang J. Complete recovery with the chain of survival after a prolonged (120 Minutes) out-of-hospital cardiac arrest due to Brugada syndrome: a case report. Medicine (Baltimore). 2015;94(27):e1107.
Matsumoto H, Umakoshi K, Kikuchi S, Uemura S, Takahashi K, Takeba J, Ohboshi M, Aibiki M. Full recovery case after 82 minutes out-of-hospital cardiac arrest: importance of chain of survival and predicting outcome. Ther Hypothermia Temp Manag. 2015;5(1):17–8.
Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, Horn J, Nolan JP, Rossetti AO, Soar J. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European resuscitation council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779–89.
Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S465–82.
Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, Herren H, Jasti J, Kudenchuk PJ, Scales DC, Stub D, Richardson DK, Zive DM. Resuscitation outcomes consortium.. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–35.
Zanyk-McLean K, Sawyer KN, Paternoster R, Shievitz R, Devlin W, Swor R. Time to awakening is often delayed in patients who receive targeted temperature management after cardiac arrest. Ther Hypothermia Temp Manag. 2016. [Epub ahead of print]
Gold B, Puertas L, Davis SP, Metzger A, Yannopoulos D, Oakes DA, Lick CJ, Gillquist DL, Holm SY, Olsen JD, Jain S, Lurie KG. Awakening after cardiac arrest and post resuscitation hypothermia: are we pulling the plug too early? Resuscitation. 2014;85(2):211–4.
Grossestreuer AV, Abella BS, Leary M, Perman SM, Fuchs BD, Kolansky DM, Beylin ME, Gaieski DF. Time to awakening and neurologic outcome in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84(12):1741–6.
Rosman L, Whited A, Lampert R, Mosesso VN, Lawless C, Sears SF. Cardiac anxiety after sudden cardiac arrest: severity, predictors and clinical implications. Int J Cardiol. 2015;181:73–6.
McCrae CS, Roth AJ, Ford J, Crew EC, Conti JB, Berry RB, Sears SF. Sleep, psychosocial functioning, and device-specific adjustment in patients with implantable cardioverter defibrillators (ICDs). Behav Sleep Med. 2016;14(1):49–66.
Cutitta KE, Woodrow LK, Ford J, Shea J, Fischer A, Hazelton G, Sears SF. Shocktivity: ability and avoidance of daily activity behaviors in ICD patients. J Cardiopulm Rehabil Prev. 2014;34(4):241–7.
Sears SF, Hazelton AG, St Amant J, Matchett M, Kovacs A, Vazquez LD, Fairbrother D, Redfearn S, Hanisch D, Dubin A, Cannon BC, Fishbach P, Kanter R, Bryant RM. Quality of life in pediatric patients with implantable cardioverter defibrillators. Am J Cardiol. 2011;107(7):1023–7.
Cullen NK, Crescini C, Bayley MT. Rehabilitation outcomes after anoxic brain injury: a case-controlled comparison with traumatic brain injury. PM R. 2009;1(12):1069–76.
Cullen NK, Weisz K. Cognitive correlates with functional outcomes after anoxic brain injury: a case-controlled comparison with traumatic brain injury. Brain Inj. 2011;25(1):35–43.
Smania N, Avesani R, Roncari L, Ianes P, Girardi P, Varalta V, Gambini MG, Fiaschi A, Gandolfi M. Factors predicting functional and cognitive recovery following severe traumatic, anoxic, and cerebrovascular brain damage. J Head Trauma Rehabil. 2013;28(2):131–40.
Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, Lang E, Cocchi MN, Xanthos T, Callaway CW, Soar J. ILCOR ALS task force. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation and the American Heart Association emergency cardiovascular care committee and the council on cardiopulmonary, critical care, perioperative and resuscitation. Circulation. 2015;132(25):2448–56.
Amorim E, Rittenberger JC, Baldwin ME, Callaway CW, Popescu A. Post cardiac arrest service. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–32.
Lu-Emerson C, Khot S. Neurological sequelae of hypoxic-ischemic brain injury. NeuroRehabilitation. 2010;26(1):35–45.
Pinto SM, Kandt JA, Ferimer S, Wagner AK. Donepezil and zolpidem associated emergence from disordered consciousness after cardiac arrest: implications involving striatal circuitry and dopamine modulation. Accepted Association of Academic Physiatrists. 2017.
Howell K, Grill E, Klein AM, Straube A, Bender A. Rehabilitation outcome of anoxic-ischaemic encephalopathy survivors with prolonged disorders of consciousness. Resuscitation. 2013;84(10):1409–15.
Donnino MW, Rittenberger JC, Gaieski D, Cocchi MN, Giberson B, Peberdy MA, Abella BS, Bobrow BJ, Callaway C. The development and implementation of cardiac arrest centers. Resuscitation. 2011;82(8):974–8.
Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998;352(9143):1808–12.
Fischer C, Luauté J, Némoz C, Morlet D, Kirkorian G, Mauguière F. Improved prediction of awakening or nonawakening from severe anoxic coma using tree-based classification analysis. Crit Care Med. 2006;34(5):1520–4.
Akahane M, Tanabe S, Koike S, Ogawa T, Horiguchi H, Yasunaga H, Imamura T. Elderly out-of-hospital cardiac arrest has worse outcomes with a family bystander than a non-family bystander. Int J Emerg Med. 2012;5(1):41.
Sasson C, Haukoos JS, Bond C, Rabe M, Colbert SH, King R, Sayre M, Heisler M. Barriers and facilitators to learning and performing cardiopulmonary resuscitation in neighborhoods with low bystander cardiopulmonary resuscitation prevalence and high rates of cardiac arrest in Columbus, OH. Circ Cardiovasc Qual Outcomes. 2013;6(5):550–8.
Barr Jr GC, Rupp VA, Hamilton KM, Worrilow CC, Reed 3rd JF, Friel KS, Dusza SW, Greenberg MR. Training mothers in infant cardiopulmonary resuscitation with an instructional DVD and manikin. J Am Osteopath Assoc. 2013;113(7):538–45.
Lasiter S, Oles SK, Mundell J, London S, Khan B. Critical care follow-up clinics: a scoping review of interventions and outcomes. Clin Nurse Spec. 2016;30(4):227–37.
Garcia SF, Kircher SM, Oden M, Veneruso A, McKoy JM, Pearman T, Penedo FJ. Survivorship care planning in a comprehensive cancer center using an implementation framework. J Community Support Oncol. 2016;14(5):192–9.
El Saghir NS, Farhat RA, Charara RN, Khoury KE. Enhancing cancer care in areas of limited resources: our next steps. Future Oncol. 2014;10(12):1953–65.
Dulko D, Pace CM, Dittus KL, Sprague BL, Pollack LA, Hawkins NA, Geller BM. Barriers and facilitators to implementing cancer survivorship care plans. Oncol Nurs Forum. 2013;40(6):575–80. doi:10.1188/13.ONF.575-580.
De Fusco P, Chlebowski R. An assessment of breast cancer survivorship awareness and educational needs among health care professionals. J Clin Oncol. 2009;27(15_suppl):e17542.
Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between cerebral performance category, modified Rankin scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–40.
Kalmar K, Giacino JT. The JFK coma recovery scale—revised. Neuropsychol Rehabil. 2005;15(3–4):454–60.
Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85(12):2020–9.
Berkhoff M, Donati F, Bassetti C. Postanoxic alpha (theta) coma: a reappraisal of its prognostic significance. Clin Neurophysiol. 2000;111(2):297–304.
Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75(3):239–45.
Whyte J, Nakase-Richardson R. Disorders of consciousness: outcomes, comorbidities, and care needs. Arch Phys Med Rehabil. 2013;94(10):1851–4.
Whyte J, Nakase-Richardson R, Hammond FM, McNamee S, Giacino JT, Kalmar K, Greenwald BD, Yablon SA, Horn LJ. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research traumatic brain injury model systems. Arch Phys Med Rehabil. 2013;94(10):1855–60.
Nora G, Harun R, Fine D, Grobart A, Munoz MJ, Hutchison DF, Kochanek PM, Drabek T, Leak R, Wagner AK. In vivo striatal characterization of dopamine neurotransmission deficits after ventricular fibrillation cardiac arrest using fast-scan cyclic voltammetry. 2016. In Review.
Calabresi P, Centonze D, Bernardi G. Cellular factors controlling neuronal vulnerability in the brain: a lesson from the striatum. Neurology. 2000;55:1249–55.
Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–24.
Gittis AH, Kreitzer AC. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012;35:557–64.
Chatelle C, Thibaut A, Gosseries O, Bruno MA, Demertzi A, Bernard C, Hustinx R, Tshibanda L, Bahri MA, Laureys S. Changes in cerebral metabolism in patients with a minimally conscious state responding to zolpidem. Front Hum Neurosci. 2014;8:917.
Daniele A, Panza F, Greco A, Logroscino G, Seripa D. Can a positive allosteric modulation of GABAergic receptors improve motor symptoms in patients with Parkinson’s disease? The potential role of zolpidem in the treatment of Parkinson’s disease. Parkinsons Dis. 2016;2016:2531812.
Kobayashi K, Higashima M, Mutou K, Kidani T, Tachibana O, Yamashita J, Koshino Y. Severe delirium due to basal forebrain vascular lesion and efficacy of donepezil. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28(7):1189–94.
Bruera E, Strasser F, Shen L, Palmer JL, Willey J, Driver LC, Burton AW. The effect of donepezil on sedation and other symptoms in patients receiving opioids for cancer pain: a pilot study. J Pain Symptom Manag. 2003;26(5):1049–54.
Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27(5):885–7.
Stocker L, Jellestad L, Jenewein J, Boettger S. Challenges in the management of delirium: a case of augmentation with donepezil following inadequate response and adverse effects with risperidone. Psychiatr Danub. 2015;27(1):64–6.
Slatkin N, Rhiner M. Treatment of opioid-induced delirium with acetylcholinesterase inhibitors: a case report. J Pain Symptom Manag. 2004;27(3):268–73.
Slatkin NE, Rhiner M, Bolton TM. Donepezil in the treatment of opioid-induced sedation: report of six cases. J Pain Symptom Manag. 2001;21(5):425–38.
Pape TL, Rosenow JM, Steiner M, Parrish T, Guernon A, Harton B, Patil V, Bhaumik DK, McNamee S, Walker M, Froehlich K, Burress C, Odle C, Wang X, Herrold AA, Zhao W, Reda D, Mallinson T, Conneely M, Nemeth AJ. Placebo-controlled trial of familiar auditory sensory training for acute severe traumatic brain injury: a preliminary report. Neurorehabil Neural Repair. 2015;29(6):537–47.
Schnakers C, Magee WL, Harris B. Sensory stimulation and music therapy programs for treating disorders of consciousness. Front Psychol. 2016;7:297.
Levy A, Chen R. Myoclonus: pathophysiology and treatment options. Curr Treat Options Neurol. 2016;18(5):21.
Davies SE, Rhys M, Voss S, Greenwood R, Thomas M, Benger JR. Psychological wellbeing in survivors of cardiac arrest, and its relationship to neurocognitive function. Resuscitation. 2016;111:22–5.
Green CR, Botha JA, Tiruvoipati R. Cognitive function, quality of life and mental health in survivors of our-of-hospital cardiac arrest: a review. Anaesth Intensive Care. 2015;43(5):568–76.
Lilja G, Nilsson G, Nielsen N, Friberg H, Hassager C, Koopmans M, Kuiper M, Martini A, Mellinghoff J, Pelosi P, Wanscher M, Wise MP, Östman I, Cronberg T. Anxiety and depression among out-of-hospital cardiac arrest survivors. Resuscitation. 2015;97:68–75.
Weeke P, Jensen A, Folke F, Gislason GH, Olesen JB, Andersson C, Fosbøl EL, Larsen JK, Lippert FK, Nielsen SL, Gerds T, Andersen PK, Kanters JK, Poulsen HE, Pehrson S, Køber L, Torp-Pedersen C. Antidepressant use and risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Clin Pharmacol Ther. 2012;92(1):72–9.
Ford J, Rosman L, Wuensch K, Irvine J, Sears SF. Cognitive-behavioral treatment of posttraumatic stress in patients with implantable cardioverter defibrillators: results from a randomized controlled trial. J Trauma Stress. 2016;29(4):388–92.
Irvine J, Firestone J, Ong L, Cribbie R, Dorian P, Harris L, Ritvo P, Katz J, Newman D, Cameron D, Johnson S, Bilanovic A, Hill A, O’Donnell S, Sears Jr S. A randomized controlled trial of cognitive behavior therapy tailored to psychological adaptation to an implantable cardioverter defibrillator. Psychosom Med. 2011;73(3):226–33.
Tazopoulou E, Miljkovitch R, Truelle JL, Schnitzler A, Onillon M, Zucco T, Hawthorne G, Montreuil M. Rehabilitation following cerebral anoxia: an assessment of 27 patients. Brain Inj. 2016;30(1):95–103.
Fitzgerald A, Aditya H, Prior A, McNeill E, Pentland B. Anoxic brain injury: clinical patterns and functional outcomes. A study of 93 cases. Brain Inj. 2010;24(11):1311–23.
Sundaram MB, Avram D, Cziffer A. Unilateral ischaemic optic neuropathy following systemic hypotension. J R Soc Med. 1986;79(4):250.
Schaadt AK, Schmidt L, Kuhn C, Summ M, Adams M, Garbacenkaite R, Leonhardt E, Reinhart S, Kerkhoff G. Perceptual relearning of binocular fusion after hypoxic brain damage: four controlled single-case treatment studies. Neuropsychology. 2014;28(3):382–7.
Sudden Cardiac Arrest Association. http://www.suddencardiacarrest.org/aws/SCAA/pt/sp/home_page. Accessed 1 Jan 2017.
Sudden Cardiac Arrest Foundation. http://www.sca-aware.org/. Accessed 1 Jan 2017.
Take Heart America. http://takeheartamerica.org/. Accessed 1 Jan 2017.
Heart Rescue Project. http://www.heartrescueproject.com/. Accessed 1 Jan 2017.
SaveMiHeart. http://www.savemiheart.org/. Accessed 1 Jan 2017.
Parent Heart Watch. https://parentheartwatch.org/. Accessed 1 Jan 2017.
Sawyer KN, Brown F, Christensen R, Damino C, Newman MM, Kurz MC. Surviving sudden cardiac arrest: a pilot qualitative survey study of survivors. Ther Hypothermia Temp Manag. 2016;6(2):76–84.
Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118(18):1854–63.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Kelly N. Sawyer, Clifton W. Callaway, and Amy K Wagner declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Traumatic Brain Injury Rehabilitation
Rights and permissions
About this article
Cite this article
Sawyer, K.N., Callaway, C.W. & Wagner, A.K. Life After Death: Surviving Cardiac Arrest—an Overview of Epidemiology, Best Acute Care Practices, and Considerations for Rehabilitation Care. Curr Phys Med Rehabil Rep 5, 30–39 (2017). https://doi.org/10.1007/s40141-017-0148-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-017-0148-7