Abstract
The goal of cervical epidural corticosteroid injections is to ameliorate pain from cervical radiculopathy. Controversy remains regarding the use and optimal delivery route of epidural medications in the cervical spine. The purpose of this review article is to compare the risks and benefits associated with cervical interlaminar and transforaminal epidural injections, and to describe alternatives to their use.
Similar content being viewed by others
Introduction
The majority of patients with cervical radiculopathy can be managed conservatively, with approximately 10–20 % eventually having surgery [1, 2]. Recurrence rates as high as 30 % are observed in patients treated both surgically and conservatively [2, 3]. The primary goal of conservative treatment of acute radicular pain is to ameliorate the symptoms and improve function while the favorable natural history runs its course. For this purpose, epidural corticosteroids in various forms have proven beneficial [4–8]. These injections are commonly used to treat radicular pain from disc herniation and degenerative stenosis. Failure of non-invasive conservative care with continued functional limitations is a relative indication for this treatment.
There are two commonly used approaches for cervical epidural injections: interlaminar and transforaminal. An interlaminar injection (occasionally called “interspinous” or inappropriately called “translaminar” or “intralaminar”) accesses the epidural space via a posterior approach between the lamina. The first description of cervical interlaminar epidural injections appeared 80 years ago [9].
Transforaminal injections in the cervical spine were more recently developed as a more target-selective alternative, and initially were presumed to provide advantages for safety and effectiveness [10–12]. A cervical transforaminal injection requires placement of a needle into the neuroforamen from an anterolateral approach. By targeting the anterior epidural space at a single level, transforaminal injections do provide more localized medication delivery [13, 14], and they are particularly target specific for foraminal and extraforaminal disc herniations [15, 16].
Debate continues on the ideal cervical epidural injection approach. This dispute arises from issues surrounding effectiveness and safety. The comparative effectiveness of these two approaches has never been determined in a prospective randomized trial, and each technique risks similar minor and serious complications [10, 17••, 18–20, 21•, 22–29]. Despite the absence of evidence for a superior technique, authors have voiced strong opinions on which injection to use in practice [30]. This chapter will discuss and compare the known risks of each approach, outline the currently published evidence for their clinical use, and review available alternatives.
Cervical Transforaminal Injection Risks
The story of cervical transforaminal injections over the past quarter century contains important lessons for physicians evaluating new treatments. In the beginning, interventionalists were happy to adopt a new cervical epidural approach since the serious risks inherent to cervical interlaminar epidural injections were well known by the time this new approach entered the scene. Early promotion of cervical transforaminal injections as a safer and more target selective alternative rapidly impacted practice patterns [31]. In the following years, reports of serious morbidity and death dispelled all myths about the improved safety of this approach [23, 25]. Many abandoned the procedure while others continued only after initiating additional safety measures.
In the hands of experts, minor complications occur in a small percentage of cases—from 0.32 to 1.6 % in two studies, each involving more than 1,000 injections [18, 32]. Reported serious complications involve different central nervous system infarctions resulting in anterior spinal artery syndrome [19, 20], quadriplegia [22], ischemic stroke [23, 24], and death [25, 26]. A recent literature review and a survey of pain physicians each highlighted the occurrence of these serious events [21•, 33]. Still, their actual incidences remain unknown since none have been recorded in any of the large published series [18, 32, 34]. Initially, different mechanisms were postulated to explain these observed neurologic infarcts. Needle-induced vessel trauma with dissection or spasm was initially suggested [19, 22], and a single case of arterial dissection was documented in a post-mortem examination [26]. Over time, evidence grew supporting another primary mechanism—inadvertent injection of particulate corticosteroids into a radiculomedullary artery or the vertebral artery resulting in microembolic ischemia [20, 24, 25, 35]. Studies of vascular anatomy demonstrate abundant collateral circulation making vasospasm of a single radicular artery an unlikely culprit [36], microscopic measures of corticosteroid particle size demonstrated the potential for an embolic shower infarction at the arteriole level [25, 27], and observations from two independent animal studies confirmed the presence of microembolic ischemia caused by intra-arterial injection of certain corticosteroids [37, 38••]. Thus, efforts to maximize the safety of cervical transforaminal injections have centered on reducing the risk of inadvertent intravascular injection.
Vascular uptake of contrast appears in one third of cervical transforaminal injections [39•, 40•] with the highest frequency observed in the upper cervical levels [40•]. Fortunately, the vast majority of these observations involve intravenous injection. Rates of inadvertent arterial injection are not as well documented with few published estimates. Radicular artery uptake was observed in 2/354 injections in one center, for an incidence of 0.56 % [41•]. Incidental vertebral artery injection was estimated to have an incidence of 0.016 % in a purely informal analysis [30].
Although the risk associated with arterial injection exceeds that of venous injections, all vascular injections should be avoided. The neuroforamen is a dangerous and costly site for an IV injection of corticosteroids, not to mention the resulting loss of diagnostic accuracy and therapeutic potential. It is also important to note that arterial injections, in some instances, may only be recognized by the subsequent venous washout and therefore mistaken as a more benign venous injection [42].
Cervical Transforaminal Risk Reduction
Several methods have been proposed to avoid inadvertent vascular injection, and investigations have shown some to be more beneficial than others. Table 1 summarizes the evidence specific to cervical transforaminal injections. Detecting potential vascular injections was an early area of focus. Aspiration for blood with a syringe was shown to have good specificity but regrettably low sensitivity [43]. Therefore, it is useful if positive, but not if negative. Image confirmation of contrast distribution can also improve safety. Using intermittent fluoroscopy to identify cervical injection contrast patterns caused experienced physicians to miss nearly 50 % of the inadvertent vascular uptake that was observed using live fluoroscopy [44]. So, careful observation of the dynamic spread of contrast under live fluoroscopy is required to maximize the detection of accidental vascular injections. In another center, digital subtraction angiography (DSA) was shown to increase the rate of vascular detection from 18 to 33 % [39•]. If the above techniques fail, the routine use of an anesthetic test dose appears to be safe and capable of uncovering potentially dangerous intravascular injections, with a positive test in just 0.56 % of injections in one multicenter study [41•].
Needle selection and placement are also important to consider. At different times short-bevel and blunt-tip needles were recommended as potentially safer alternatives [45–47]. A later prospective study revealed no advantages in vascular injection risk using short-bevel needles [48•]. Animal studies have demonstrated that large-gauge blunt-tip needles are less likely than sharp needles to enter blood vessels and produce bleeding [46, 49], but this advantage was less noticeable with blunt-tip needles in sizes that are more likely to be used in the spine (22 and 25-gauge). This finding was confirmed in a prospective study of patients undergoing lumbar transforaminal injections that found no difference in inadvertent vascular injection rates between the sharp beveled needles, whitacre needles and blunt-tip needles [50]. At one time larger gauge needles were considered less likely to result in inadvertent vascular injection, however even the small radicular arteries can be cannulated by a 22-gauge needle [51]. Furthermore, needle gauge does not impact the observed vascular rates in cervical injections [40•], although it did impact the type of vascular injections observed. Specifically, larger gauge needles are more likely to produce vascular contrast patterns that appear simultaneous to the expected epidural injection [40•]. Since this specific type of vascular pattern is more difficult for physicians to detect [44], this finding favors the use of smaller gauge needles.
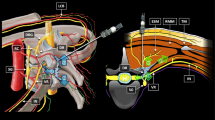
Needle placement in the posterior foramen is intended to avoid the vertebral artery, yet this region is not always safe. Radiographic studies show that intraforaminal vertebral artery anomalies are present in 7.6 % of patients [52•], and this can place the vertebral artery in the intended path of the needle [36]. Thus, a careful review of advanced imaging is advised before placing a needle for a cervical transforaminal injection. While shallow placement in the foramen is sometimes considered, a cadaveric study showed that radicular arteries are larger and thus more likely to be cannulated in the outer foramen [36]. Plus, other deep cervical arteries with occasional feeder branches to the radicular arteries are more likely located here. As a result, the ideal depth of needle placement remains the mid-foramen (Fig. 1).
Left image is an AP fluoroscopic view of a left C6–C7 transforaminal injection with epidural and peri-radicular contrast displacement. The image also shows ideal needle depth with the tip of the needle at the mid-point between two imaginary vertical lines. The first imaginary line corresponds with the lateral edge of the lateral pillar and the second with the uncinate line. Image on the right is from a C7–T1 interlaminar injection. This image demonstrates the contralateral oblique view of needle tip placed into the epidural space along with epidural contrast displacement
Currently, some safety methods are based on common sense alone, including patient screening for dissection risk factors, minimizing sedation, and avoiding needle manipulation after contrast confirmation by injection through extension tubing [25, 53]. Manipulating a needle to change from a contrast-filled syringe to a corticosteroid-filled syringe risks moving the needle tip. Accidental needle movement, even very small distances that are undetectable by the human hand, can be sufficient to move the needle from outside to inside a vessel lumen. Thus, the results of a contrast injection may not apply to the distribution of medications injected after swapping syringes on the needle hub. To avoid this, an extension tube is connected to the needle hub thru which all medications are injected without manipulating the needle. Although procedural competency is difficult to measure objectively, it is common sense that practitioners must ensure they have adequate training and experience to perform these potentially dangerous injections [54]. Indeed, one study did show that experience was positively associated with accuracy of detecting incidental vascular injections [54].
In the end, the ability to prove risk reduction by each of these methods is limited by the extremely low incidence of these complications. An extremely large prospective trial will be required to demonstrate the effectiveness of any single safety method. Ethical concerns limit the feasibility of such a trial. Recently, a case report questioned the accuracy of some of these tests by detailing a case of paraplegia following a lumbar transforaminal epidural injection performed using several of the above safety measures [55•].
Thus, as a final measure of safety, dexamethasone has become the corticosteroid of choice for cervical transforaminal injections. Since its particles are smaller than red blood cells [25, 56], it can avoid microembolic ischemia even if injected intra-arterially [37, 38••]. Further support for using dexamethasone in cervical transforaminal epidural injections comes from a small prospective randomized trial comparing it to a commonly used large-particulate corticosteroid with no significant differences in outcomes between the two treatment groups [57].
Cervical Interlaminar Injection Risks
To date, the largest study of cervical epidural injection risk is a retrospective survey of International Spine Intervention Society instructors who reported on a combined 5,968 cervical epidural steroid injections, 73 % interlaminar and 27 % transforaminal [18]. While the overall complication rate was higher in the interlaminar group (0.52 vs. 0.32 %), the difference was not statistically significant. No serious complications were reported in either group. This study’s retrospective nature likely underestimates the true incidence of complications. Another retrospective chart audit of 345 interlaminar epidural injections also uncovered only minor and transient complications, but with a higher overall rate of 16.8 % [58]. This difference is explained by the inclusion of several complications not reported in the larger study. The specific minor complications reported and their incidences include: neck pain (6.7 %), non-positional headache (4.6 %), insomnia (1.7 %), vasovagal reaction (1.7 %), facial flushing (1.5 %), dural puncture (0.3 %), and fever the night of the procedure (0.3 %). These side effects and complications are similar to those seen in lumbar interlaminar injections.
Cervical interlaminar epidural injections have a time-honored track record, more than three times that of cervical transforaminal injections. Consequently, serious complications resulting from cervical interlaminar epidural injections have long been recognized. Published reports document cases of epidural hematoma with various neurologic involvement [59•, 60, 61], spinal cord injury [62], and death [29]. Closed-claims data demonstrates that a large number of unreported cases also exist [17••]. The actual incidence of these events is unknown. Here again, none have been reported in a large published case series [18], but their continued appearance is one factor that led many to use the transforaminal approach when it was introduced.
Cervical interlaminar injections are most often administered at the C6–C7 or C7–T1 interspaces because the dorsal epidural space above this is scant [63, 64•, 65]. Unfortunately, lateral imaging of the lower cervical spine can be obscured by shoulder anatomy, making it difficult to confirm the needle tip position. This has resulted in inadvertent needle advancement and injection into the spinal cord producing a permanent myelopathy [62]. A study of cervical injections for chronic pain in the American Society of Anesthesiologists’ closed-claims database found that direct needle trauma and injection during interlaminar epidural injections was responsible for a greater number of spinal cord injuries than any other mechanism, including incidental arterial injection during transforaminal procedures [17••]. Interestingly, the spinal cord injured patients were fve times more likely to be unresponsive from sedation during the procedure.
Like transforaminals, interlaminar injections also have a vascular mechanism underlying potential catastrophic injuries, however the exact mechanisms differ. Excluding a major vascular malformation, the dorsal epidural space is void of arterial communication to central nervous system tissues, so arterial injection is not a major concern. What is of concern is the creation of a symptomatic hematoma [59•, 60, 61, 66, 67]. Hematomas are more commonly reported in patients receiving continuous instead of single-shot epidural anesthesia [68]. Currently, there are no published estimates of the risk of epidural hematoma from single-shot epidural anesthetics, or in patients receiving epidural corticosteroid injections.
Even with proper technique and optimal needle placement, an epidural hematomas can occur following an interlaminar injection. On the other hand, needle placement in the cervical foramen has not been linked to symptomatic epidural hematoma formation. Certainly foraminal venous bleeding must occur in some transforaminal injections. Possibly, excess blood in the foramen flows preferentially into the extraforaminal tissues instead of building pressure in the spinal epidural space, whereas bleeding in the dorsal epidural space has no such escape. Because of this, some consider transforaminal injections a better option in any patient with increased bleeding risk, although here caution is also warranted.
Cervical Interlaminar Risk Reduction
Risk mitigation strategies for interlaminar epidural injections have focused on reducing epidural bleeding and maximizing needle visualization. With percutaneous epidural access, there are two known risk factors for a spinal hematoma: continuous access for medication infusions, and disordered clotting. No other procedure or patient-related details are known to play a role, including needle type and gauge, practitioner experience, midline versus paramedian approach, etc. Thus, screening for and managing potential bleeding risk factors remains the only safety measure to minimize hematoma risk. To this end, the American Society for Regional Anesthesia and Pain Medicine has produced specific guidelines for treatment of patients undergoing neuraxial anesthetic procedures [69••]. It is beyond the scope of this chapter to outline these risk factors and their recommended management, however it is important to note that hematomas can occur even when these guidelines are closely followed [70•].
Cervical interlaminar injections are ideally performed using image guidance. In the study of closed-claims data, the on-site reviewers indicated that the appropriate use of radiographic guidance would have prevented the spinal cord injury in 45 % of cases [17••]. Traditionally a lateral view is used to monitor needle depth, however shoulder anatomy can obscure this view. To improve visualization of needle tip depth and avoid inadvertent intradural and intramedullary placement, alternate imaging strategies were recently described. Depending upon the specific needle orientation and trajectory, the “contralateral oblique view” (Fig. 1) may provide superior information regarding needle depth [71••]. Currently, many experienced practitioners are adopting this approach.
A few additional items deserve mention. The blood aspiration test during interlaminar injections, as with transforaminal injections, can produce false negative results [72, 73]. Excessive sedation should be avoided since a minimally responsive patient cannot provide the feedback that might indicate contact with the dura and its contents [17••, 64•, 74]. Some even argue that patients should not be sedated during these injections [74]. Finally, as with transforaminal injections, a careful review of advanced imaging before the procedure is useful to evaluate the available dorsal epidural space and to help plan the optimal needle trajectory.
Comparative Benefits and Risks
Relative to lumbar radiculopathy, cervical radiculopathy treatments are poorly researched and thus more controversial. In the lumbar spine, evidence suggests that epidural injections are effective in reducing pain and need for surgery. In addition, randomized controlled trials demonstrate that outcomes of lumbar transforaminal injections are superior to lumbar interlaminar injections [11, 12]. Currently, the efficacy of cervical epidural injections, both interlaminar and transforaminal, remains unknown because neither has been tested against a placebo. Additionally, these two approaches have never been directly compared so their comparative effectiveness is unknown. Although the evidence is limited, it is not completely lacking.
There is one published randomized trial on cervical interlaminar injections. This study compared them to intramuscular corticosteroid injections, with superior results observed in the interlaminar injection group [8]. This finding supports the theory that a more target specific and higher concentration of corticosteroids produces better results. Of concern to some is that interlaminar injections are often delivered at sites distal to the known pathology, reducing their target specificity. Still, some studies have shown that just 2 cc of contrast can spread from the lower cervical spine up to the C2 level [75, 76]. In routine practice, compared to transforaminals, interlaminar epidurals involve larger injectate volumes containing less concentrated corticosteroids. Carried to its logical conclusion, the target specific theory would suggest a preference for transforaminal injections. While evidence supports this argument in the lumbar spine [11, 12], it has never been demonstrated in the cervical spine.
To overcome the lack of site specificity with interlaminar injections, an epidural catheter can be inserted and passed thru the epidural space to a target site [77]. Since interlaminar epidural injections have been shown to fail to deliver medications to the site of pathology in up to 74 % of patients [78] this practice seems logical on the surface. Regrettably, it also increases the risks inherent to interlaminar injections. Passage of a catheter provides additional opportunity for trauma to epidural veins and increases hematoma risk, much like continuous epidural access for medication infusions. Plus, multiple prospective studies have demonstrated that the standard interlaminar approach is effective in reducing pain and need for surgeries [8, 79–82, 83••].
For transforaminal epidural injections there are two published randomized trials. The first compared results of injections using small particle to large particle corticosteroids with equal benefits in the two groups [57]. The second study compared transforaminal injections of anesthetic and corticosteroid to transforaminal injections with anesthetic and saline [84]. While the authors concluded that no differences were observed between the two groups, there are serious problems with this study. The methods used to obtain the outcomes information is not defined, and the outcomes questionnaire used to measure success is not described. Thus, it is difficult to draw any conclusions from this study. As a result, the current best evidence comes from several prospective cohort studies. These have consistently demonstrated clinical benefits following cervical transforaminal injections [53, 83••, 85]. In one study, the majority of patients recovered after a single injection [85].
When a decision is made to proceed with a cervical epidural injection, selecting between an interlaminar and transforaminal approach can be challenging. A proper risk–benefit analysis requires a comparison of both the effectiveness and the risks. Since comparative effectiveness data is lacking in the cervical spine, the risk–benefit profile rests on the risk side of the equation. Unfortunately, a clear decision cannot be made here either. Studies comparing risks associated with these two injections have found no significant differences [18]. Serious morbidity and death are rare complications documented with each procedure [17••, 30]. The incidence of these major complications remains unknown so any claim that one procedure is safer than the other is utterly unfounded. What is clear is that research during the past several years has focused more on improving the safety of transforaminal injections, but this does not guarantee superior safety relative to interlaminar injections. Because of these known risks, combined with the short supply of evidence, epidural injections are not considered first-line treatment for cervical radiculopathy. One must first consider the alternatives.
Alternatives
Fortunately, the natural history of cervical radiculopathy is favorable. The majority of individuals improve within several weeks or months without the aid of epidural injections [1]. Time spent educating and counseling patients about the expected course is of great value. It relieves fears and helps patients understand the benefits of a conservative treatment approach.
Medical management often starts with nonsteroidal anti-inflammatory drugs. Evidence of their effectiveness for neck pain relief is sparse [86, 87], and they do carry risks of renal, cardiovascular and gastrointestinal complications [88–90]. Opioid pain medications and muscle relaxants are known to help in the short-term [91, 92]. However, they produce drowsiness in approximately one third of patients, and can impair driving [93]. Long-term use of opioids is highly controversial. Many other pain-modifying drugs have been used in clinical practice, including tricyclic antidepressants and anti-epileptic drugs. More recently, duloxetine has received an FDA indication for osteoarthritis pain so its use is on-label for radiculopathy stemming from degenerative spine disease.
Systemic corticosteroids, oral and parenteral, are sometimes used. Surprisingly, this practice has never been shown to benefit patients with cervical radiculopathy. As detailed above, intramuscular corticosteroid administration is inferior to interlaminar epidural injection based on a single randomized trial [8]. Another well-designed placebo controlled randomized trial found that oral corticosteroids were no better than placebo in the treatment of lumbar radiculopathy [94]. No other evidence exists to support or refute the use of systemic corticosteroids.
Other alternatives include physical therapy, mobilization, traction and acupuncture. Here again, data is sparse [95]. A randomized clinical trial of patients with chronic cervical radiculopathy compared surgery to physical therapy and cervical collar treatments finding no differences in pain, muscular strength or sensory loss at 4 months [96, 97]. A systematic Cochrane review of mobilization for neck pain found that mobilization combined with exercise is beneficial for persistent mechanical neck disorders, however there was insufficient evidence to draw conclusions for cervical radiculopathy [98]. Cervical traction is occasionally used as a supplement to manual therapy and exercise, however results from a multicenter randomized trial showed no additional benefit when traction was added to a multimodal treatment program [99]. Acupuncture is frequently used but there is no clear demonstration of its effectiveness in cervical radiculopathy [100].
Overall, the literature regarding non-invasive treatments for cervical radiculopathy is lacking. In fact, the evidence is less than that supporting epidural injection. Given the known favorable course of the disease, and the low risks associated with these alternative treatments, they still deserve first consideration. Epidural injections should be reserved for patients with the most severe symptoms who fail an initial course of more conservative treatment.
Conclusion
Current evidence regarding the conservative and interventional treatment of cervical radiculopathy provides limited guidance. While evidence supporting epidural corticosteroid injections exceeds that for non-invasive conservative measures, the risks associated with epidural injections and the generally favorable course of cervical radiculopathy support initial use of the conservative measures. While cervical epidural injections have a low incidence of minor complications, severe morbidity and death are rare complications associated with existing techniques, transforaminal and interlaminar. Currently, an evidence-based argument cannot be made to support the selection of one injection approach over the other. Methods to improve the safety of each approach have been described and studied, and practitioners need to understand their respective utility and limits.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Saal JS, Saal JA, Yurth EF. Nonoperative management of herniated cervical intervertebral disc with radiculopathy. Spine. 1996;21:1877–83.
Radhakrishnan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117:325–35.
Gore DR, Sepic B. Anterior discectomy and fusion for painful cervical disc disease: a report of 50 patients with an average follow-up of 21 years. Spine. 1998;23:2047–51.
Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, Goette K. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82A(11):1589–93.
Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg Am. 2004;86A(4):670–9.
Lin EL, Lieu V, Halevi L, Shamie AN, Wang JC. Cervical epidural steroid injections for symptomatic disc herniations. J Spinal Disord Tech. 2006;19(3):183–6.
Bush K, Hillier S. A controlled study of caudal epidural injections of triamcinolone plus procaine for the management of intractable sciatica. Spine. 1991;16:572–5.
Stav A, Ovadia L, Sternberg A, et al. Cervical epidural steroid injection for cervicobrachialgia. Acta Anaesthesiol Scand. 1993;37:562–6.
Dogliotti AM. Research and clinical observations on spinal anesthesia: with special reference to the peridural technique. Anesth Analg. 1933;12(2):59–65.
Windsor RE, Storm S, Sugar R, Nagula D. Cervical transforaminal injection: review of the literature, complications, and a suggested technique. Pain Physician. 2003;6(4):457–65.
Schaufele MK, Hatch L, Jones W. Interlaminar versus transforaminal epidural injections for the treatment of symptomatic lumbar intervertebral disc herniations. Pain Physician. 2006;9:361–6.
Thomas E, Cyteval C, Abiad L, et al. Efficacy of transforaminal versus interspinous corticosteroids injection in discal radiculalgia—a prospective, randomized, double-blind study. Clin Rheumatol. 2003;22:299–304.
Bogduk N, Aprill C, Derby R. Epidural steroid injections. In: White AH, editor. Spine care operative treatment. St. Louis: Mosby; 1995. p. 322–42.
Woodard J, Herring S, Windsor R, et al. Epidural procedures in spine pain and management. In: Lennard T, editor. Physiatric procedures in clinical practice. Philadelphia: Hanky and Belfus; 1995. p. 260–91.
Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine. 1992;17:S176–83.
Weiner BK, Fraser RD. Foraminal injection for lateral lumbar disc herniation. J Bone Joint Surg Br. 1997;79:804–7.
•• Rathmell JP, Michna E, Fitzgibbon DR, Stephens LS, Posner KL, Domino KB. Injury and liability associated with cervical procedures for chronic pain. Anesthesiology. 2011;114(4):918–26. Caution is advised in using heavy sedation and general anesthesia during cervical procedures, as spinal cord injury is more common in those patients.
Derby R, Lee SH, Kim BJ, et al. Complications following cervical epidural steroid injections by expert interventionalists in 2003. Pain Physician. 2004;7:445–9.
Brouwers PJ, Kottink EJ, Simon MA, Prevo RL. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91(3):397–9.
Rosenkranz M, Grzyska U, Niesen W, et al. Anterior spinal artery syndrome following periradicular cervical nerve root therapy. J Neurol. 2004;251:229–31.
• Benny B, Azari P, Briones D. Complications of cervical transforaminal epidural steroid injections. Am J Phys Med Rehabil 2010;89:601–7. Serious complications can occur with cervical transforaminal epidural injection. Proposed methods to reduce these risks are outlined.
Bose B. Quadriparesis following cervical epidural steroid injections: case report and review of the literature. Spine J. 2005;5:558–63.
Scanlon G, Romanowsky S, Schulteis G, Wallace M. Neurological infarctions following cervical transforaminal epidural steroid injections. Pain Med. 2005;6(2):177–8.
Beckman WA, Mendez RJ, Paine GF, Mazzilli MA. Cerebellar herniation after cervical transforaminal epidural injection. Reg Anesth Pain Med. 2006;31(3):282–5.
Tiso R, Cutler T, Catania J, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4(4):468–74.
Rozin L, Rozin R, Koehler SA, et al. Death during transforaminal epidural steroid nerve root block (C7) due to perforation of the left vertebral artery. Am J Forensic Med Pathol. 2003;24:351–5.
Williams KN, Jackowski A, Evans PJ. Epidural haematoma requiring surgical decompression following repeated cervical epidural steroid injections for chronic pain. Pain. 1990;42:197–9.
Huang RC, Shapiro GS, Lim M, et al. Cervical epidural abscess after epidural steroid injection. Spine. 2004;29:E7–9.
Reitman CA, Watters W 3rd. Subdural hematoma after cervical epidural steroid injection. Spine. 2002;27:E174–6.
Smuck M, Rosenberg JM, Akuthota V. The use of epidural corticosteroids for cervical radiculopathy: an interlaminar versus transforaminal approach. PMR. 2009;1(2):178–84.
Manchikanti L. Comment on cervical epidural steroid injection with intrinsic spinal cord damage. Spine. 1999;24:1170–2.
Ma DJ, Gilula LA, Riew KD. Complications of fluoroscopically guided extraforaminal cervical nerve blocks. An analysis of 1036 injections. J Bone Joint Surg Am. 2005;87:1025–30.
Scanlon G, Moeller-Bertram T, Romanowsky S, Wallace M. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine. 2007;32:1249–56.
Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86:277–83.
Rathmell JP, Aprill C, Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004;100:1595–600.
Huntoon MA. Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain. 2005;117(1–2):104–11.
Okubadejo GO, Talcott MR, Schmidt RE, et al. Perils of intravascular methylprednisolone injection into the vertebral artery. An animal study. J Bone Joint Surg Am. 2008;90:1932–8.
•• Dawley JD, Moeller-Bertram T, Wallace MS, Patel PM. Intra-arterial injection in the rat brain: evaluation of steroids used for transforaminal epidurals. Spine. 2009;34(16):1638–43. In vivo animal models demonstrated that intra-arterial injection of traditionally used depot steroids results in central nervous system injury. Intra-arterial injections of small particle steroids did not demonstrate this complication.
• McLean JP, Sigler JD, Plastaras CT, et al. The rate of detection of intravascular injection in cervical transforaminal epidural steroid injections with and without digital subtraction angiography. PMR. 2009;1(7):636–42. Real-time fluoroscopic visualization of contrast injection using digital subtraction angiography (DSA) nearly doubles the rate of intravascular detection during cervical transforaminal epidural steroid injections in one center.
• Smuck M, Tang CT, Fuller BJ. Incidence of simultaneous epidural and vascular injection during cervical transforaminal epidural injections. Spine. 2009;34(21):E751–5. Simultaneous epidural and vascular injection accounts for over half of all vascular injections observed during cervical transforaminal epidural injections, thus vascular injections can be missed unless live fluoroscopy is used during contrast injection.
• Smuck M, Maxwell MD, Kennedy D, et al. Utility of the anesthetic test dose to avoid catastrophic injury during cervical transforaminal epidural injections. Spine J. 2010;10(10):857–64. The routine use of an anesthetic test dose appears to be safe and capable of detecting potentially dangerous intravascular injections undetected by conventional techniques.
Karasek M, Bogduk N. Temporary neurologic deficit after cervical transforaminal injection of local anesthetic. Pain Med. 2004;5:202–5.
Furman MB, Giovanniello MT, O’Brien EM. Incidence of intravascular penetration in transforaminal cervical epidural steroid injections. Spine. 2003;28:21–5.
Smuck M, Fuller B, Chiodo A, et al. Accuracy of intermittent fluoroscopy to detect intravascular injection during transforaminal epidural injections. Spine. 2008;33:E205–10.
Derby R, Baker R, Dreyfuss P, Weinstein SM. Cervical radicular pain: transforaminal vs interlaminar steroid injections. SpineLine. 2004;5:16–21.
Nelson J. Letter to the editor Re: Blunt needles. Int Spine Interv Soc Newsl. 2006;5:58–60.
Bogduk N. Sharp vs. blunt needles. International Spine Intervention Society white paper. Interv Spine. 2005;5:7–13.
• Smuck M, Yu A, Tang C, Zemper E. Influence of needle type on the incidence of intravascular injection during transforaminal epidural injections: a comparison of short-bevel and long-bevel needles. Spine J. 2010;10(5):367–71. Needle type does not alter the incidence of inadvertent vascular injections during lumbar transforaminal epidural injections.
Akins EW, Hawkins IF Jr, Mladinich C, et al. The blunt needle: a new percutaneous access device. AJR Am J Roentgenol. 1989;152:181–2.
Patel A, Martinez-Ith A, Smuck M. A prospective analysis of alternative needles for lumbosacral transforaminal epidural injections. Pain Med. 2012;13(8):1103.
Hoeft MA, Rathmell JP, Monsey RD, Fonda BJ. Cervical transforaminal injection and the radicular artery: variation in anatomical location within the cervical intervertebral foramina. Reg Anesth Pain Med. 2006;31(3):270–4.
• Eskander MS, Drew JM, Aubin ME, et al. Vertebral artery anatomy: a review of two hundred fifty magnetic resonance imaging scans. Spine. 2010;35(23):2035–40. Intraforaminal vertebral artery anomalies are present in 7.6% of patients. This can increase the risk of vertebral artery injection during cervical transforaminal injections, even with proper needle placement.
Derby R. Point of view: cervical epidural steroid injection with intrinsic spinal cord damage: two case reports. Spine. 1998;23(19):2141–2.
Smuck M, Abbott Z, Zemper E. Interpretation of contrast dispersal patterns by experienced and inexperienced interventionalists. PMR. 2009;1(1):55–9.
• Chang Chien GC, Candido KD, Knezevic NN. Digital subtraction angiography does not reliably prevent paraplegia associated with lumbar transforaminal epidural steroid injection. Pain Physician. 2012;15(6):515–23. A case of paraplegia associated with a lumbar transoframinal epidural injection questions the reliability of many current methods to reduce risks during these injections.
Derby R, Lee SH, Date ES, Lee JH, Lee CH. Size and aggregation of corticosteroids used for epidural injections. Pain Med. 2008;9(2):227–34.
Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006;7:237–42.
Botwin KP, Castellanos R, Rao S, et al. Complications of fluoroscopically guided interlaminar cervical epidural injections. Arch Phys Med Rehabil. 2003;84(5):627–33.
• Landa J, Kim Y. Outcomes of interlaminar and transforminal spinal injections. Bull NYU Hosp Jt Dis. 2012;70(1):6–10. This topical review highlights research demonstrating that both transforaminal and interlaminar epidural corticosteroid injections can provide reliable pain relief for up to 6 months.
Stoll A, Sanchez M. Epidural hematoma after epidural block: implication for its use in pain management. Surg Neurol. 2002;57:235–40.
Ghaly RF. Recovery after high-dose methylprednisolone and delayed evacuation: a case of spinal epidural hematoma. J Neurosurg Anesthesiol. 2001;13:323–8.
Abbasi A, et al. Complications of interlaminar cervical epidural steroid injections. A review of the literature. Spine. 2007;32(19):2144–51.
Abbasi A, Malhotra G, Malanga G, et al. Complications of interlaminar cervical epidural steroid injections: a review of the literature. Spine. 2007;32:2144–51.
• Tamayo AC, Guajardo-Rosas J, Hernandez-Ortiz A. Cervical epidural injections for radicular pain. Tech Reg Anesth Pain Manag. 2010;14:106–12. Catastrophic complications from cervical transforaminal epidural steroid injections are believed to be secondary to arterial occlusion by particulate steroids.
Goel A, Pollan JJ. Contrast flow characteristics in the cervical epidural space: an analysis of cervical epidurograms. Spine. 2006;31:1576–9.
Markham JW, Lynge HN, Stahlman GE. The syndrome of spontaneous spinal epidural hematoma. Report of three cases. J Neurosurg. 1967;26:334–41.
Yagi S, Hida K, Iwasaki Y, Abe H, et al. Cervical epidural hematoma caused by cervical twisting after epidural anesthesia: a case report. No Shinkei Geka. 1998;26:235–42.
Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26(1):1–49.
•• Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (third edition). Reg Anesth Pain Med. 2010;35(1):64–101. During cervical epidural injections spinal hematoma is a rare and potentially catastrophic complication with increased risk in patients on antithrombotic therapy. Minimizing these risks is essential. This is the most comprehensive treatment guideline published on this topic.
• Xu R, Bydon M, Gokaslan ZL, et al. Epidural steroid injection resulting in epidural hematoma in a patient despite strict adherence to anticoagulation guidelines. J Neurosurg Spine. 2009;11(3):358–64. Care should be taken when performing epidural injections in patients on anticoagulation medications. As this case demonstrates, epidural hematomas can occur in these patients despite strict adherence to published guidelines.
•• Landers MH, Dreyfuss P, Bogduk N. On the geometry of fluoroscopy views for cervical interlaminar epidural injections. Pain Med. 2012;13(1):58–65. Depending upon the specific needle orientation and trajectory, the “contralateral oblique view” may provide superior information regarding interlaminar needle depth when compared to the traditional lateral view.
Ho KY. Vascular uptake of contract despite negative aspiration in interlaminar cervical epidural injection. Pain Physician. 2006;9:267–8.
Stretanski MF, Chopko B. Unintentional vascular uptake in fluoroscopically guided, contrast-confirmed spinal injections: a 1-yr clinical experience and discussion of findings. Am J Phys Med Rehabil. 2005;84(1):30–5.
Hodges SD, Castleberg RL, Miller T, et al. Steroid injection with intrinsic spinal cord damage: two case reports. Spine. 1998;23(19):2137–40.
Stojanovic MP, Vu TN, Caneris O, et al. The role of fluoroscopy in cervical epidural steroid injections: an analysis of contrast dispersal patterns. Spine. 2002;27:509–14.
Kim KS, Shin SS, Kim TS, et al. Fluoroscopically guided cervical interlaminar epidural injections using the midline approach: an analysis of epidurography contrast patterns. Anesth Analg. 2009;108(5):1658–61.
Larkin TM, Carragee E, Cohen S. A novel technique for delivery of epidural steroids and diagnosing the level of nerve root pathology. J Spinal Disord Tech. 2003;16(2):186–92.
Fredman B, Nun MB, Zohar E, et al. Epidural corticosteroids for treating “failed back surgery syndrome”: is fluoroscopy really necessary? Anesth Analg. 1999;88(2):367–72.
Bush K, Hillier S. Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J. 1996;5:319–25.
Rowlingson JC, Kirschenbaum LP. Epidural analgesic techniques in the management of cervical pain. Anesth Analg. 1986;65:938–42.
Cicala RS, Westbrook L, Angel JJ. Side effects and complications of cervical epidural steroid injections. J Pain Symptom Manage. 1989;4:64–6.
Shulman M. Treatment of neck pain with cervical epidural steroid injection. Reg Anesth. 1986;11:92.
•• Lee SH, Kim KT, Kim DH, et al. Clinical outcomes of cervical radiculopathy following epidural steroid injection: a prospective study with follow-up for more than 2 years. Spine. 2012;37(12):1041–7. In patients with cervical radiculopathy who were considered surgical candidates, diversion of care to epidural corticosteroid injections reduced pain and prevented progression to surgery in more than 80% of patients.
Anderberg L, Annertz M, Persson L, et al. Transforaminal steroid injections for the treatment of cervical radiculopathy: a prospective and randomised study. Eur Spine J. 2007;16(3):321–8.
Vallee JN, Feydy A, Carlier RY, et al. Chronic cervical radiculopathy: lateral approach periradicular corticosteroid injection. Radiology. 2001;218:886–92.
Vos C, Verhagen A, Passchier J, et al. Management of acute neck pain in general practice: a prospective study. Br J Gen Pract. 2007;57:23–8.
Binder AI. Cervical spondylosis and neck pain. BMJ. 2007;334:527–31.
Hurwitz EL, Carragee EJ, van der Velde G, et al. Treatment of neck pain: noninvasive interventions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(Suppl):S123–52.
Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomized trials. BMJ. 2006;332:1302–8.
Hernandez-Diaz S, Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–9.
Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305.
Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–14.
Guzman J, et al. Clinical practice implications of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders: from concepts and findings to recommendations. J Manipulative Physiol Ther. 2009;32(2 Suppl):S227–43. doi:10.1016/j.jmpt.2008.11.023.
Haimovic IC, Beresford HR. Dexamethasone is not superior to placebo for treating lumbosacral radicular pain. Neurology. 1986;36(12):1593–4.
Riddle DL, Schappert SM. Volume and characteristics of inpatient and ambulatory medical care for neck pain in the United States: data from three national surveys. Spine. 2007;32:132–41.
Persson LC, Carlsson CA, Carlsson JY. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar. A prospective, randomized study. Spine. 1997;22:751–8.
Persson LC, Moritz U, Brandt L, Carlsson CA. Cervical radiculopathy: pain, muscle weakness and sensory loss in patients with cervical radiculopathy treated with surgery, physiotherapy or cervical collar. A prospective, controlled study. Eur Spine J. 1997;6:256–66.
Gross AR, Hoving JL, Haines TA, et al. Manipulation and mobilisation for mechanical neck disorders. Cochrane Database Syst Rev. 2004:CD004249.
Young IA, Michener LA, Cleland JA, et al. Manual therapy, exercise, and traction for patients with cervical radiculopathy: a randomized clinical trial. Phys Ther. 2009;89:632–42.
White AR, Ernst E. A systematic review of randomized controlled trials of acupuncture for neck pain. Rheumatology. 1999;38:143–7.
Disclosure
M Smuck is a consultant with Arthocare Corp, has received a lecture honorarium and travel expenses from NASS Instructional, and his institution has a grant with Cytonics Corp; R Demirjian declares no conflicts of interest; and DJ Kennedy declares no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smuck, M., Demirjian, R. & Kennedy, D.J. Cervical Foraminal Versus Interlaminar Epidurals: Risks, Benefits, and Alternatives. Curr Phys Med Rehabil Rep 1, 125–134 (2013). https://doi.org/10.1007/s40141-013-0013-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-013-0013-2