Abstract
The use of antiretrovirals as pre-exposure prophylaxis (PrEP) is highly efficacious in HIV prevention. The World Health Organization recently recommended Truvada® (Gilead Sciences, Inc.) or tenofovir disoproxil fumarate (TDF) for high-risk individuals, with limited data for single-agent TDF PrEP in men who have sex with men (MSM). We report two cases of TDF PrEP failure in MSM who had received long-term TDF for hepatitis B infection and had therapeutic levels of drug immediately after HIV acquisition. Rapid antiretroviral intensification at diagnosis of acute HIV infection failed to limit immune dysfunction or prevent the establishment of a viral reservoir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of antiretrovirals (ARVs) as pre-exposure prophylaxis (PrEP) is highly efficacious at limiting HIV transmission [1–6]. Recent World Health Organization (WHO) guidelines recommend single-agent tenofovir disoproxil fumarate (TDF) or Truvada® [TDF/emtricitabine (FTC); Gilead Sciences, Inc.] for individuals at risk of HIV acquisition [7]. Single-agent TDF PrEP shows benefit over placebo and is comparable with Truvada in preventing HIV transmission in HIV serodiscordant heterosexual couples [2] and individuals who inject drugs [3]. Efficacy data for TDF in men who have sex with men (MSM) are limited [8] and it is not known whether drug level requirements are the same for both HIV treatment and prevention. However, animal model data suggest that TDF alone is less protective than Truvada [9, 10] and pharmacokinetic analysis of the partners PrEP study supports this [11].
Cases of PrEP failure mainly occur due to poor adherence [1–6, 11]. Early detection is essential to minimize monotherapy drug exposure and prevent drug resistance development [12]. Whether ARV therapy (ART) should be stopped or intensified at PrEP failure is unclear [13]. A recent case presentation reported an undetectable viral reservoir in an individual’s intensifying ART at PrEP failure [14]. This is supported by cohort data and data from macaques suggest that three-drug ARV at acute HIV infection can improve clinical outcome [15–17] by limiting viral reservoir [17–20] and immune dysfunction [21–23], and that these benefits are greater the earlier the ARVs are started [15–17].
We report two cases of PrEP failure amongst MSM receiving long-term TDF for hepatitis B treatment with therapeutic tenofovir levels around the time of HIV-1 acquisition. Despite continuing TDF and intensifying to combination ARV at acute HIV diagnosis, both cases had significant viral reservoirs and elevated markers of immune activation/exhaustion within 4 weeks of HIV-1 diagnosis consistent with established HIV-1 infection.
Case reports
Patients
Informed consent was obtained from both patients for being included in this case report.
Two patients from separate HIV centers were diagnosed with acute HIV whilst receiving TDF monotherapy for hepatitis B infection. To allow case comparison, day 1 was defined as the day of estimated date of HIV seroconversion (EDSC, i.e., the mid-point date between HIV-negative and HIV-positive test or 14 days prior to p24 antigen-positive/HIV antibody-negative result).
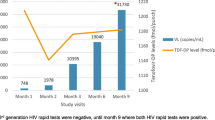
Both patients had received TDF 300 mg/day for hepatitis B infection (hepatitis B s-antigen and e-antigen positive) and maintained a consistently undetectable hepatitis B DNA for 3+ years with no viral blips. In the 6 weeks prior to HIV acquisition both individuals reported condom-less receptive anal sex with casual male partners and denied any missed doses of TDF. Longstanding good adherence was further suggested by the regularity of pharmacy script provision and drug levels carried out at HIV diagnosis were within the therapeutic range (Fig. 1). No other sexually transmitted infections were detected at HIV diagnosis. ARV was intensified within 3 weeks of presumed HIV acquisition and blood taken for quantification of viral reservoir and immune function approximately 1 week later. The cases are summarized in Table 1.
Laboratory Results
Patient A was diagnosed with acute HIV infection (HIV antibody positive 10 days after HIV antibody-negative test and by 3 bands on Western Blot) following a 4-day history of mild flu-like symptoms suggestive of seroconversion illness. Results 9 days after EDSC were: CD4 T cell count 584 cells/µL (35%), CD4:CD8 ratio 1.19, and HIV-1 plasma viral load <50 copies/mL. The ARV regimen was immediately intensified (12 days from EDCS) to Eviplera® (25 mg rilpivirine, 200 mg emtricitabine, and 245 mg TDF; Gilead Sciences International Ltd.) and the viral load remained undetectable thereafter. Viral genotype failed to amplify due to low viral load. Results 15 days after EDSC (3 days after ARV intensification) showed total HIV-1 DNA 1381 copies/million CD4 cells, integrated DNA 586.6 copies/million CD4 T cells, and unspliced intracellular HIV-1 RNA transcripts 116 copies/106 copies 18 s RNA.
Patient B was diagnosed with acute HIV (p24 antigen-positive/antibody-negative antibody) following hospitalization with a severe seroconversion illness comprising severe flu-like symptoms, fatigue, and myalgia. Results 14 days after EDSC were: CD4 T cell count 550 cells/µL (24%), CD4:CD8 ratio 0.49, and HIV-1 plasma viral load 103,306 copies/mL. The regimen was intensified 19 days after EDSC to Truvada (one tablet once daily), raltegravir (400 mg twice daily), darunavir (800 mg once daily), and ritonavir (100 mg once daily). Viral genotype showed wild-type drug-sensitive virus. Blood taken 24 days after EDSC (5 days after ART intensification) showed total HIV-1 DNA 2746 copies/million CD4 cells, integrated DNA 1431.6 copies/million CD4 T cells, and unspliced intracellular HIV-1 RNA transcripts 1236 copies/106 copies 18 s RNA.
Immune activation, defined as the percentage expression of CD38 and HLA-DR on CD4 and CD8 T cells, was 65.8 and 57% for patient A and 42.2 and 38.8% for patient B.
Methods
HIV Diagnosis, Viral Load, and Therapeutic Drug Level Monitoring
HIV testing was carried out using the Abbott Architect HIV Ag/Ab combo assay and VIDAS quantitative HIV p24 11 assay. Confirmation occurred with Vidas HIV Duo Quick (HIV6) ELFA fourth-generation assay and Bispot Immunocomb third-generation assay.
Western blot was carried out using MP Diagnostics HIV Blot 2.2. HIV-1 plasma viral load was quantified by Roche COBAS V2.0, and viral genotype determined using Taqman sequencing. Tenofovir (TFV) drug levels were measured by a validated HPLC–MS/MS with a lower limit of quantification of 5 ng/mL [24]. The tenofovir concentration for each patient was plotted against a percentile plot derived from a published population pharmacokinetic model of tenofovir plasma concentrations in HIV-infected subjects (Fig. 1) [25].
HIV Reservoir
Purified CD4 T cells were analyzed by qPCR for HIV-1 DNA (total and integrated) and cell-associated HIV-1 RNA unspliced transcripts (CA-RNA) as reported elsewhere [19].
Immune Activation and Exhaustion
PBMC were stained with the anchor markers (CD3-VioBlue, CD4(VIT4)-VioGreen, CD8-APC) and a Live/Dead marker Near IR-APC-Cy7 plus either an activation panel (CD25(3G10)-PE, CD38-PE-Vio770, CD69-FITC, Anti-HLA-DR-PerCP) or an exhaustion panel (TIGIT-PE, TIM-3-FITC, LAG-3-PerCPeF710, PD1-PE-Cy7). Cells were run on a MACSQuant and analyzed with FlowJo software v10 (Miltenyi Biotec).
Discussion
As PrEP is becoming more widely available and uptake increasing, this is a timely reminder that TDF monotherapy PrEP in MSM has limited efficacy data and that HIV-1 acquisition can occur in the presence of TFV drug levels within the therapeutic range required to treat HIV [26]. These cases are instructive to providers and the field of PrEP, and highlight that patients with HBV receiving tenofovir for HBV should consider intensification to Truvada (TDF/FTC) if they meet guidelines (e.g., Centers for Disease Control and Prevention) for PrEP. The lack of resistance detected concurs with randomized control study data showing a very low incidence of resistance in cases of tenofovir failure [29].
Although PrEP effectiveness is largely driven by adherence [1–8, 11, 26, 27], this was not implicated in these cases as evidenced by consistently undetectable hepatitis B DNA and therapeutic plasma levels of tenofovir at HIV diagnosis (a proxy for HIV acquisition). Whilst drug levels were high enough to treat both hepatitis B and HIV infection [26], the drug level required to protect from HIV infection is not known and, if higher, may explain the transmission events. Additionally, it is not known whether hepatitis B increases susceptibility to HIV and the presence of TDF-resistant mutations may need to be excluded by minor variant sequencing [12].
These cases also show that rapid intensification with ARVs did not prevent reservoir seeding or immune dysfunction. Indeed, the level of viral reservoir, immune activation, and immune exhaustion were comparable to those observed in untreated primary HIV cohorts [15]. Patient A is of additional interest as this occurred despite no evidence of on-going viral replication in plasma prior to ARV intensification. This suggests the importance of sanctuary sites for the establishment of viral reservoir and is consistent with primate models [27].
Conclusion
Single-drug TDF PrEP was not effective in preventing HIV infection and intensified ART post-infection did not reduce the viral reservoir in either patient. Whilst single-agent PrEP is not to be recommended as an HIV prevention strategy in MSM in European [28] and US [13] PrEP guidelines, it is by the WHO [7]. Close monitoring of outcomes of TDF usage in MSM needs to be carried out.
References
Grant RM, Lama JR, Anderson PL, et al. Pre-exposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. 2010;363:2587–99.
Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90.
Van Damme L, Corneli A, Ahmed K, et al. Pre-exposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22.
McCormack S Dunn D, Gafos M, et al. Pragmatic Open-Label Randomised Trial of preexposure prophylaxis: the PROUD Study. 2015 conference on retroviruses and opportunistic infections (CROI), Seattle, USA, abstract 22LB; 2015.
Molina J-M Capitant C, Spire B, et al. On demand PrEP with oral TDF-FTC in MSM: results of the ANRS Ipergay Trial. 2015 conference on retroviruses and opportunistic infections (CROI), Seattle, USA, abstract 23LB; 2015.
WHO: Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. September 2015. Available from: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed Nov 11, 2015.
Grohskopf LA, Chillag KL, Gvetadze R, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79–86.
Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–11.
Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS ONE. 2012;7(12):2012.
Baeten JM, Donnell D, Mugo NR, et al. Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2014;14(11):1055–64.
Grant RM, Liegler T, Defechereux P, et al. Drug resistance and plasma viral RNA level after ineffective use of oral pre-exposure prophylaxis in women. AIDS. 2015;29(3):331–7.
Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2014 Clinical Practice Guideline. Available from: http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf.
Hatano H, et al. Lack of detectable HIV DNA in a PrEP study participant treated during “hyperacute” HIV infection. 21st CROI. 3–6 March 2014, Boston. Late breaker poster 397LB. http://croiconference.org/sites/all/abstracts/397LB.pdf (PDF). Accessed 1202015.
Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–30.
Fidler S, Porter K, Ewings F, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207–17.
Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211.
Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS ONE. 2012;7(3):e33948.
Williams JP, Hurst J, Stöhr W, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife. 2014;3:e03821.
Whitney JB, Hill AL, Sanisetty S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512(7512):74–7.
Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12):e1004543.
Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6.
Thornhill J Inshaw J, Oomeer S, et al. Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc. 2014;17(4Suppl 3).
Jackson A, Moyle G, Watson V, et al. Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: implications for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2013;62(3):275–81.
Baheti G, Kiser JJ, Havens PL, et al. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55(11):5294–9.
Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of Tenofovir disoproxil fumarate blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340–8.
Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annu Rev Med. 2013;64:219–32.
European Guidelines for treatment of HIV-infected adults in Europe 2015. Available from: http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf. Accessed Nov 11, 2015.
Lehman DA, Baeten JM, McCoy CO, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single or dual agent preexposure prophylaxis. J Infect Dis. 2015;211(8):1211–8.
Acknowledgments
We would like to thank the Kings College London Infectious Diseases Biobank. No funding was received for publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Julie Fox has received research grants from ViiV and Gilead and has done a Jansen BHIVA symposium. Saye Khoo has received research funding support from Merck, Gilead, Janssen, and ViiV. David Back has received an educational grant from Gilead, and honoraria from Gilead for lectures and Advisory Boards. Michael Brady, Hannah Alexander, Olubanke Davies, Nicola Robinson, Mathew Pace, Laura Else, John Cason, Sarah Fidler, and John Frater have nothing to disclose.
Compliance with Ethics Guidelines
Informed consent was obtained from both patients for being included in this case report.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of CHERUB Collaboration.
J. Frater and S. Fidler are joint senior authors and contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fox, J., Brady, M., Alexander, H. et al. Tenofovir Disoproxil Fumarate Fails to Prevent HIV Acquisition or the Establishment of a Viral Reservoir: Two Case Reports. Infect Dis Ther 5, 65–71 (2016). https://doi.org/10.1007/s40121-015-0102-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-015-0102-x