Abstract
This research addresses a detailed study on the sensitivity and selectivity of ZnO thin film to volatile organic compound (VOC) vapors that can be used for the development of VOC sensors. The ZnO thin film of 100 nm thickness was prepared by post-annealing of e-beam evaporated Zn thin film. The sample was structurally, morphologically, and chemically characterized by X-ray diffraction and field emission scanning electron microscopy analyses. The sensitivity, selectivity, and detection limit of the sample were tested with respect to a wide range of common VOC vapors, including acetone, formaldehyde, acetic acid, formic acid, acetylene, toluene, benzene, ethanol, methanol, and isopropanol in the temperature range of 200–400 °C. The results show that the best sensitivity and detection limit of the sample are related to acetone vapor in the studied temperature range. The ZnO thin film-based acetone sensor also shows a good reproducibility and stability at the operating temperature of 280 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volatile organic compound or VOC is the name given to a substance that contains carbon and evaporates at room temperature. VOCs are widely used in household and commercial products such as cleansers, disinfectants, waxes, glues, cosmetics, dry cleaning products, paints, varnishes and preservatives, gasoline, kerosene, and other fuels [1]. The ability of VOCs to cause harmful health effects varies greatly [1, 2]. As with other chemical vapors, the effects of VOC exposure depends on several factors including the type of VOC, the amount of VOC, and the length of time a person is exposed [1, 2]. There are also remarkable correlations between VOC emissions and different kinds of cancers [2, 3]. Although VOCs can be found in both outdoor and indoor settings, the levels of VOCs found indoors can be much higher than those found outdoors (up to 10 times higher), and it is estimated that 50–300 different VOCs may be detected in the air of homes, schools, offices, and commercial buildings at any given time [4]. Therefore, the development of a reliable and low-cost VOC sensor is highly necessary. Compared to conventional techniques, low-dimensional nanostructured ZnO due to the remarkable chemical, physical, and sensing properties [5,6,7] can be a good candidate to detect the VOCs [3, 8,9,10,11,12]. Many researchers have been reported the study of different volatile vapors sensing properties of nano-structured ZnO but it seems that there is a lack of detailed and more deeply study on VOCs sensing properties of nano-structured ZnO. For example, Al-Hardan et al. [3] produced ZnO thin films using the RF technique and heat treated them at 500 °C for 6 h. They studied the sensitivity of the ZnO thin films to ethanol, isopropanol, and acetone. Muthukrishnan et al. [9] used the sol–gel dip coating method to obtain ZnO thin films and investigated the sensitivity of the sample to acetone, ethanol, and acetaldehyde. Luo et al. [10] employed a hydrothermal method to produce mesoporous ZnO and Sn-doped ZnO thin films and studied the sensitivity of the samples to different volatile vapors including ethanol, methanol, acetone, formaldehyde, and ammonia. Peng et al. [8] also prepared 3D flower-like hierarchical ZnO nanostructure by the hydrothermal method and investigated the sensitivity of the sample to different gases and vapors such as acetone, hydrogen, acetic acid, ethanol, methanol, formaldehyde, and toluene. In contrast to the previous works and to obtain a deep understanding of the VOC sensing properties of nanostructured ZnO, we focused on sensitivity, selectivity and detection limit of the ZnO thin film with respect to a wide range of common VOCs including acetone, formaldehyde, acetic acid, formic acid, acetylene, toluene, benzene, ethanol, methanol, and isopropanol. In addition to these, we studied the reliability of the ZnO thin film-based VOC sensor.
Experimental details
In previous research, we studied the sensitivity of the ZnO thin films to hydrogen and methane gases and optimized the sensitivity of the ZnO thin films with respect to the deposition method [6], post-annealing conditions [7], as well as thickness [13]. Based on the knowledge obtained from our previous works, Zn thin film of 100 nm thickness was deposited on SiO2/Si substrate by the e-beam evaporation technique and then post-annealed in a horizontal tube furnace at 500 °C with an oxygen flow of 200 standard cubic centimeter per minute (sccm) for 60 min. Details of the substrate preparation, deposition condition, and post-annealing processes are given in Ref. [7].
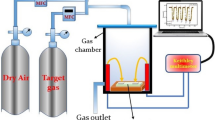
The sample was structurally, morphologically, and chemically characterized by means of a Philips X-ray diffractometer (X’pert multi-purpose diffractometer (MPD); CuKa radiation) with a step size of 0.02° and step time of 1 s, and field emission scanning electron microscopy (FESEM) (CamScan MV2300, Czech and England). To fabricate the gas-sensing element based on ZnO thin film, a pair of Au electrodes with dimensions of 3 × 3 mm2 was deposited on the ZnO thin film by the e-beam evaporation method using a mask made to be used in this application. To measure the response of the sample, it was positioned in a domestic-made chamber and the electrical resistance of the sample was measured in dry air and in the presence of a wide range of VOC vapors including acetone (C3H6O), formaldehyde (CH2O), acetic acid (CH3COOH), formic acid (CH2O2), acetylene (C2H2), toluene (C7H8), benzene (C6H6), ethanol (C2H5OH), methanol (CH3OH), and isopropanol (C3H7OH) in the temperature range of 200–400 °C. Schema and setup of the gas sensing measurement system and the method of calculating the concentration and injection volume of vapors are given in [7, 10], respectively. The main parameters which will be used to investigate the sensing properties are also listed below:
-
Response (S) is defined as the ratio of R a/R g (S = R a/R g), where R a and R g are the resistance of the metal oxide semiconductors in the air and the presence of volatile vapors, respectively [8].
-
Response or recovery time was estimated as the time taken for the sensor output to reach 90% of its saturation after applying or switching off the target gas in a step function [8].
-
Selectivity of a sensor is defined as the ratio of its response to a certain object to that of other objects as K = S A/S B. S A and S B are the responses of a sensor to a target gas (gas A) and an interfering gas (gas B), respectively [14].
-
Detection limit is the lowest amount of the object/vapor that the sensor can detect [6].
-
Reproducibility of a sensor is evaluated by the change of sensing behavior after switching numerous times between the ‘ON’ state and ‘OFF’ state [14].
-
Stability is a parameter to evaluate the feature of a sensor to maintain its properties when it is continuously employed in hostile environments for a long time [12].
Results and discussion
Figure 1 depicts the X-ray diffraction (XRD) pattern of the ZnO thin film prepared in this research. The diffraction lines are matched very well with those given by the JCPDS Card no.: 36–1451 attributed to a hexagonal wurtzite structure. It can be distinguished easily that the (002) is a preferred orientation and the produced sample has a large fraction of (002) facets. The chemical composition of the ZnO thin film is obtained by energy dispersive X-ray (EDX) analysis and the EDX spectrum of the sample is shown in Fig. 2. The result shows that the ratio of O to Zn is 1.15 and confirms the ZnO structure. Figure 3 depicts the FESEM micrograph of the ZnO thin film prepared in this work. It can be seen that the sample shows a structure of nanosheets. A detailed study of the dependence of crystallographic structure, chemical composition, and surface morphology on the deposition method and the post-annealing process has been given in our previous research [5,6,7].
To obtain the sensitivity of the sample produced in this work for the detection of different VOC vapors mentioned in the previous sections, the electrical resistance was measured in air and in the presence of 100 ppm concentration of different VOC vapors in the temperature range of 200–400 °C. The response of the sample to various VOCs at different temperatures is given in Table 1, while a bar chart of this data is shown in Fig. 4. It is noteworthy that the highest response value for various vapors is obtained at different temperatures in the following order: formaldehyde (11) at 360 °C, acetic acid (11) at 320 °C, acetylene (8) at 360 °C, formic acid (7) at 360 °C, toluene (8) at 360 °C, benzene (7) at 360 °C, acetone (30) at 280 °C, ethanol (14) at 320 °C, methanol (10) at 360 °C, and isopropanol (23) at 280 °C. This behavior can be due to the fact that the energy of adsorption, reaction, and desorption of various vapors on the film’s surface is different. From this data, it can also be deduced that the most sensitivity is attributed to acetone vapor at different temperatures; however, it becomes maximum at the operating temperature of 280 °C. Figure 5 depicts the selectivity of the ZnO thin film to acetone relative to the other vapors at the operating temperature of 280 °C. It can be seen that the ZnO thin film prepared in this work shows a remarkable selectivity to acetone vapor with respect to other VOCs. To explain these behaviors, we start with reviewing of the VOC vapors sensing mechanism of the ZnO thin film. When ZnO is exposed to air, oxygen molecules are chemisorbed on the surface of the film in various forms such as \({\text{O}}_{2}^{ - }\), \({\text{O}}^{ - }\), or \({\text{O}}^{2 - }\) ions by capturing electrons from the conduction band. Transfer of electrons from the conduction band reduces the free carrier density and causes the generation of the electron depletion region at the surface of the film, which in turn increases the resistance of the ZnO film [15]. After that, the ZnO thin film (an n-type metal oxide semiconductor) is exposed to VOC vapors (reducing vapors), the adsorbed vapor reacts with the chemisorbed oxygen anions on the film surface, and then oxygen ions release the trapped electrons back to the conduction band, which result in the decrease of sensor resistance. It is shown in the XRD analysis that the produced sample has a large fraction of (002) facets, and it is well known that all of the Zn atoms at (002) the surface of the hexagonal ZnO are unsaturated coordinated, exhibiting the most dangling bonds. These dangling bonds result in the predominant exposed polar (002) facets that are highly polarized and contain more of the above-mentioned oxygen vacancies [8, 16]. On the other hand, acetone compared with the other VOCs investigated in this work has the largest dipole moment (Table 2) which in turn facilitates the adsorption and the reaction processes with polar (002) facets at lower temperature [8].
To investigate the detection limit, the ZnO thin film was exposed to VOC vapors with different concentrations in the range of 5–400 ppm at the attributed operating temperature and the best responses were obtained in the following order: 2.5 for 20 ppm of formaldehyde at 360 °C, 2 for 20 ppm of acetic acid at 320 °C, 4 for 50 ppm of acetylene at 360 °C, 3 for 50 ppm of formic acid at 360 °C, 4 for 50 ppm of toluene at 360 °C, 3 for 50 ppm of benzene at 360 °C, 2 for 10 ppm of acetone at 280 °C, 3 for 20 ppm of ethanol at 320 °C, 2.5 for 20 ppm of methanol at 360 °C, and 5 for 20 ppm of isopropanol at 280 °C. From these investigations it can be concluded that the ZnO thin film-based sensor prepared in this research shows the best sensitivity, selectivity, and detection limit to the acetone vapor compared with the other VOC vapors studied in this work. Hence, we focus on the acetone sensing performance of the ZnO thin film-based sensor.
Figure 6 depicts the dynamic variations of the response of the ZnO thin film exposed to acetone vapor with different concentrations (10–300 ppm) at the operating temperature of 280 °C. The response, response time, and recovery time as a function of acetone concentration obtained from the curves in Fig. 6 are shown in Fig. 7. It can be observed that an increase in acetone concentration causes the increase in the response and decrease in the response time. It is quite logical that the increase of acetone concentration provides more vapor atoms to react with the ZnO film surface per unit time and as a result changes in resistance are more and fast. However, it is expected that the sensor becomes saturated when all of the surface atoms react with vapor atoms.
Details of published works on acetone gas sensitivity of the ZnO thin films prepared by various methods [3, 8, 15, 18,19,20,21] and the results of this research are given in Table 3. A comparison of the results shows many differences between the produced samples by various methods; however, it seems that the sample prepared in this work may be a good candidate as an acetone sensor. The differences between the results listed in Table 3 can be due to the different factors such as the growth or preparation method and conditions, substrate, thickness, and electrode which in turn affect the crystallinity, porosity, surface morphology, as well as sensing mechanism.
In addition to pure ZnO, there are many reports on acetone vapor sensitivity of other metal oxide semiconductors such as WO3 [22], α-Fe2O3 [23], In2O3 [24], SnO2 [25], and Fe2O4 [26] as well as doped-ZnO [18, 27, 28], and a summary of their results is listed in Table 4. From the results given in this work and Tables 3 and 4, it may be also deduced that ZnO is suitable to detect acetone vapor compared to the other metal oxide semiconductors.
To study the reproducibility of the ZnO thin film-based acetone sensor, the response of the sample was tested to 100 ppm of acetone at an operating temperature of 280 °C three times continuously. The results of this test are plotted in Fig. 8 and show a good reproducibility of the sample. The stability of the ZnO thin film-based acetone sensor was also investigated. To this end, the response of the sample was tested to 100 ppm of acetone at an operating temperature of 280 °C as a function of day in steps of 3 days for 21 days (Fig. 9). It can be seen that the response of the sample is decreased with the time lapse from 30 to 26 (namely, about 87% stability) and then found a steady state. The mentioned reduction of response may be attributed to the reaction of the film surface with its surroundings between and during the measurements, especially humidity [14].
It is also worth mentioning that the sensing property measurements in this research were carried out in dry air ,while the sensitivity of the ZnO-based sensor can be affected by relative humidity (RH). The effect of relative humidity on the sensitivity of the ZnO-based gas sensor can be also found in the literature [29,30,31].
Conclusion
ZnO thin films of 100 nm thickness with a hexagonal wurtzite crystallographic structure and (002) preferred orientation was prepared according to the knowledge obtained from the previous research, namely deposition by the e-beam evaporation method on the SiO2/Si substrate and then post-annealing at 500 °C with an oxygen flow of 200 sccm for 60 min. The sensitivity and selectivity of the sample were optimized with respect to different VOC vapors and the operating temperature. The detection limit of the sample was also investigated in the range of 5–400 ppm. The sample showed the best sensitivity, selectivity as well as detection limit to the acetone vapor compared with the other vapors in the temperature range of 200–400 °C, whereas the mentioned parameters were optimum at the operating temperature of 280 °C. The ZnO thin film-based acetone sensor also showed a remarkable reproducibility and about 87% stability at the operating temperature of 280 °C.
References
Bloemen, H.J.Th., Burn, J.: Chemistry and analysis of volatile organic compounds in the environment. Chapman & Hall (1993)
Boeglin, M.L., Wessels, D., Henshel, D.: An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ. Res. 100, 242–254 (2006)
Al-Hardan, N.H., Abdullah, M.J., Abdul Aziz, A., Ahmad, H., Low, L.Y.: ZnO thin films for VOC sensing applications. Vac. 85, 101–106 (2010)
Tiwary, A., Colls, J.: Air Pollution: Measurement, Modelling and Mitigation. CRC, Boca Raton (2009)
Khojier, K., Savaloni, H., Amani, E.: Influence of annealing conditions on the crystallographic structure, chemical composition and luminescence of ZnO thin films. Appl. Surf. Sci. 289, 564–570 (2014)
Teimoori, F., Khojier, K., Dehnavi, N.Z.: Investigation on the electrical and methane gas-sensing properties of ZnO thin films produced by different methods. J. Elec. Mater. 45, 4881–4889 (2016)
Khojier, K., Savaloni, H.: Improving the H2 gas sensitivity of ZnO thin films by modifying the annealing conditions. J. Elec. Mater. 44, 3458–3464 (2015)
Peng, C., Guo, J., Yang, W., Shi, C., Liu, M., Zheng, Y., Xu, J., Chen, P., Huang, T., Yang, Y.: Synthesis of three-dimensional flower-like hierarchical ZnO nanostructure and its enhanced acetone gas sensing properties. J. Alloy. Compd. 654, 371–378 (2016)
Muthukrishnan, K., Vanaraja, M., Boomadevi, S., Karn, R.K., Singh, V., Singh, P.K., Pandiyan, K.: Studies on acetone sensing characteristics of ZnO thin film prepared by sol-gel dip coating. J. Alloy. Compd. 673, 138–143 (2016)
Luo, S., Shen, Y., Wu, Z., Cao, M., Gu, F., Wang, L.: Enhanced ethanol sensing performance of mesoporous Sn-doped ZnO. Mater. Sci. Semicond. Process. 41, 535–543 (2016)
Wei, S., Wang, S., Zhang, Y., Zhou, M.: Different morphologies of ZnO and their ethanol sensing property. Sens. Actuators B 192, 480–487 (2014)
Mirzaei, A., Leonardi, S.G., Neri, G.: Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review. Ceram. Int. 42, 15119–15141 (2016)
Teimoori, F., Khojier, K., Dehnavi, N.Z.: On the dependence of H2 gas sensitivity of ZnO thin films on film thickness. Procedia Mater. Sci. 11, 474–479 (2015)
Zolghadr, S., Khojier, K., Kimiagar, S.: Study of sensitivity and selectivity of α-Fe2O3 thin films for different toxic gases and alcohols. Mater. Sci. Semicond. Process. 54, 6–13 (2016)
Bian, H., Ma, S., Sun, A., Xu, X., Yang, G., Yan, S., Gao, J., Zhang, Z., Zhu, H.: Improvement of acetone gas sensing performance of ZnO nanoparticles. J. Alloy. Compd. 658, 629–635 (2016)
Tian, S., Yang, F., Zeng, D., Xie, C.: Solution-processed gas sensors based on ZnO nanorods array with an exposed (0001) facet for enhanced gas-sensing properties. J. Phys. Chem. C 116, 10586–10591 (2012)
Dean, J.A.: Lange’s Handbook of Chemistry. McGraw-Hill, New York (1985)
Prajapati, C.S., Sahay, P.P.: Influence of In doping on the structural, optical and acetone sensing properties of ZnO nanoparticulate thin films. Mater. Sci. Semicond. Process. 16, 200–210 (2013)
Zeng, Y., Zhang, T., Yuan, M., Kang, M., Lu, G., Wang, R., Fan, H., He, Y., Yang, H.: Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B 143, 93–98 (2009)
Li, Y., Li, D.L., Liu, J.C.: Optical and gas sensing properties of mesoporous hollow ZnO microspheres fabricated via a solvothermal method. Chin. Chem. Lett. 26, 304–308 (2015)
Kakati, N., Jee, S.H., Kim, S.H., Oh, J.Y., Yoon, Y.S.: Thickness dependency of sol-gel derived ZnO thin films on gas sensing behaviors. Thin Solid Films 519, 494–498 (2010)
Kim, S., Park, S., Park, S., Lee, C.: Acetone sensing of Au and Pd-decorated WO3 nanorod sensors. Sens. Actuators B 209, 180–185 (2015)
Kaneti, Y.V., Moriceau, J., Liu, M., Yuan, Y., Zakaria, Q., Jianga, X., Yu, A.: Hydrothermal synthesis of ternary α-Fe2O3–ZnO–Au nano composites with high gas-sensing performance. Sens. Actuators B 209, 889–897 (2015)
Vomiero, A., Bianchi, S., Comini, E., Faglia, G., Ferroni, M., Poli, N., Sberveglieri, G.: In2O3 nano wires for gas sensors: morphology and sensing characterization. Thin Solid Films 515, 8356–8359 (2007)
Mei, L., Deng, J., Yin, X., Zhang, M., Li, Q., Zhang, E., Xu, Z., Chen, L., Wang, T.: Ultra sensitive ethanol sensor based on 3D aloe-like SnO2. Sens. Actuators B 166–167, 7–11 (2012)
Khandekar, M.S., Tarwal, N.L., Mulla, I.S., Suryavanshi, S.S.: Nanocrystalline Ce doped CoFe2O4 as an acetone gas sensor. Ceram. Int. 40, 447–452 (2014)
Li, X., Chang, Y., Long, Y.: Influence of Sn doping on ZnO sensing properties for ethanol and acetone. Mater. Sci. Eng., C 32, 817–821 (2012)
Liu, L., Li, S., Zhuang, J., Wang, L., Zhang, J., Li, H., Li, H., Liu, Z., Han, Y., Jiang, X., Zhang, P.: Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuators B 155, 782–788 (2011)
Santra, S., Guha, P.K., Ali, S.Z., Hiralal, P., Unalan, H.E., Covington, J.A., Amaratunga, G.A.J., Milne, W.I., Gardner, J.W., Udrea, F.: ZnO nanowires grown on SOI CMOS substrate for ethanol sensing. Sens. Actuators B 146, 559–565 (2010)
Santra, S., De Luca, A., Bhaumik, S., Ali, S.Z., Udrea, F., Gardner, J.W., Ray, S.K., Guha, P.K.: Dip pen nanolithography-deposited zinc oxide nanorods on a CMOS MEMS platform for ethanol sensing. RSC Adv. 5, 47609–47616 (2015)
Rakshit, T., Santra, S., Manna, I., Ray, S.K.: Enhanced sensitivity and selectivity of brush-like SnO2 nanowire/ZnO nanorod heterostructure based sensor for volatile organic compounds. RSC Adv. 4, 36749–36756 (2014)
Acknowledgements
This work was carried out with the support of the Islamic Azad University, Central Tehran branch. The authors are grateful to Dr. Arman Toni for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Teimoori, F., Khojier, K. & Dehnavi, N.Z. Investigation of sensitivity and selectivity of ZnO thin film to volatile organic compounds. J Theor Appl Phys 11, 157–163 (2017). https://doi.org/10.1007/s40094-017-0253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-017-0253-0