Abstract
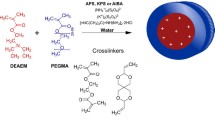
Glioblastoma multiforme is a fast-growing malignant brain tumor with poor prognosis and low survival rate. Here we investigated the potential use of anticancer drug dioxadet loaded into nanogels for glioma treatment. We used block copolymer of polyethylene glycol and polymethacrylic acid for synthesis of dioxadet carriers and two types of cross-linking agents: non-degradable ethylenediamine and biodegradable cystamine, containing disulfide bond. We analyzed physicochemical properties of nanoparticles, their loading capacity, cytotoxicity of drug-loaded nanogels, and also their internalization into glioma cells. We found the optimal conditions that promote the efficient loading of the drug. We demonstrated that dioxadet loaded nanogels have relatively high level of loading capacity (>35% w/w) and loading efficiency (>75%). We shown that nanogels with the biodegradable cross-links prone to dissociate under reducing conditions (glutathione) that allow to decrease IC50 values of the drug compared to the nanogels with ethylendiamine cross-links. This stimuli-sensitive behavior of nanogels could be beneficial for tumor treatment. Confocal analysis of glioma cells demonstrated that both types of nanogels accumulate in cells and localize in lysosomes. These results indicate that loading of dioxadet into nanoparticles can improve its performance; such formulation has a potential for further studies and practical applications.





Similar content being viewed by others
References
Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K (2005) Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem 16:122–130. doi:10.1021/bc0498166
Bennis S, Chapey C, Couvreur P, Robert J (1994) Enhanced cytotoxicity of doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres against multidrug-resistant tumour cells in culture. Eur J Cancer 30:89–93. doi:10.1016/S0959-8049(05)80025-5
Bespalov VG, Zhabin AA, Stukov AN, Beljaeva OA, Murazov JaG, Semenov AL, Kon’kov SA, Krylova IM (2013) Synergism of antitumor action of dioxadet and cisplatin in model of ascitic ovarian tumor. Sibirskij Onkol J 55:42–46 (in Russian)
Bodley AL, Liu LF, Israel M, Ramakrishnan S, Yoshihiro K, Giuliani FC, Kirschenbaum S, Silber R, Potmesil M (1989) DNA topoisomerase II-mediated interaction of doxorubicin and daunorubicin congeners with DNA. Cancer Res 49:5969–5978
Bontha S, Kabanov AV, Bronich TK (2006) Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Control Release 114:163–174. doi:10.1016/j.jconrel.2006.06.015
Bronich TK, Nehls A, Eisenberg A, Kabanov VA, Kabanov AV (1999) Novel drug delivery systems based on the complexes of block ionomers and surfactants of opposite charge. Coll Surf B Biointerfaces 16:243–251. doi:10.1016/S0927-7765(99)00075-2
Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV (2005) Polymer micelle with cross-linked ionic core. J Am Chem Soc 12:8236–8237. doi:10.1021/ja043042m
Fuertes MA, Castilla J, Alonso C, Perez JM (2003) Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 10:257–266. doi:10.2174/0929867033368484
Horwitz SB (1994) Taxol (paclitaxel): mechanisms of action. Ann Oncol 5(Suppl 6):S3–S6
Jones RB, Matthes S, Shpall EJ et al (1993) Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine. J Natl Cancer Inst 85:640–647. doi:10.1093/jnci/85.8.640
Kabanov AV, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed 48:5418–5429. doi:10.1002/anie.200900441
Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS (2000) Doxorubicin-loaded poly(ethylene glycol)–poly(b-benzyl-l-aspartate copolymer micelles: their pharmaceutical characteristics and biological significance. J Control Release 64:143–153. doi:10.1016/S0168-3659(99)00133-9
Kato N, Hasegawa U, Morimoto N, Saita Y, Nakashima K, Ezura Y, Kurosawa H, Akiyoshi K, Noda M (2007) Nanogel-based delivery system enhances PGE2 effects on bone formation. J Cell Biochem 101:1063–1070. doi:10.1002/jcb.21160
Khiati S, Luvino D, Oumzil K, Chauffert B, Camplo M, Barthelemy P (2011) Nucleoside lipid-based nanoparticles for cisplatin delivery. ACS Nano 11:8649–8655. doi:10.1021/nn202291k
Kim JO, Kabanov AV, Bronich TK (2009) Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J Control Release 138:197–204. doi:10.1016/j.jconrel.2009.04.019
Kim JO, Sahay G, Kabanov AV, Bronich TK (2010) Polymeric micelles with ionic cores containing biodegradable cross-links for delivery of chemotherapeutic agents. Biomacromolecules 11:919–926. doi:10.1021/bm9013364
Kim JO, Ramasamy T, Yong CS, Nukolovа NV, Bronich TK, Kabanov AV (2013) Cross-linked polymeric micelles based on block ionomer complexes. Mendeleev Commun 23:179–186. doi:10.1016/j.mencom.2013.07.001
Loshadkin NA, Kurlyandskiy BA, Daryina LV (2002) Toksikologiya alkiliruyushchikh soedineniy. In: Kurlyandskiy BA, Philov VA (ed) Obshchaya toksikologiya. Meditsina, Moscow, pp 236–257 (in Russian)
Lu YJ, Wei KC, Ma CCM, Yang SY, Chen JP (2012) Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Coll Surf B 89:1–9. doi:10.1016/j.colsurfb.2011.08.001
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul 41:189–207. doi:10.1016/S0065-2571(00)00013-3
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi:10.1016/0022-1759(83)90303-4
Murakami T, Ajima K, Miyawaki J, Yudasaka M, Iijima S, Shiba K (2004) Drug-loaded carbon nanohorns: adsorption and release of dexamethasone in vitro. Mol Pharm 1:399–405. doi:10.1021/mp049928e
Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK (2011) Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 32:5417–5426. doi:10.1016/j.biomaterials.2011.04.006
Oberoi HS, Laquer FC, Marky LA, Kabanov AV, Bronich TK (2011) Core cross-linked block ionomer micelles as pH-responsive carriers for cis-diamminedichloroplatinum(II). J Control Release 153:64–72. doi:10.1016/j.jconrel.2011.03.028
Siegel R, Desantis R, Virgo C et al (2012) Cancer treatment and survivorship statistics. CA Cancer J Clin 0:1–22. doi:10.3322/caac.21149
Stukov AN, Gershanovich ML, Blank MA et al (2011) Antitumor agents. NIKA, St. Petersburg (in Russian)
Sun WT, Zhang N, Li AG, Zou WW, Xu WF (2008) Preparation and evaluation of N3-O-toluyl-fluorouracil-loaded liposomes. Int J Pharm 353:243–250. doi:10.1016/j.ijpharm.2007.11.017
Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC (2007) Complexation and release of doxorubicin from its complexes with Pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release 121:137–145. doi:10.1016/j.jconrel.2007.05.010
Tokunaga Y, Nakashima M, Shibata S, et al (1997) Antitumor effects of 4-pyridoxate diamine hydroxy platinum, a novel cisplatin derivative, against malignant gliomas in vitro and in vivo: a comparison with cisplatin. Pharm Sci 3:353–356.
Yang XY, Zhang XY, Ma YF, Huang Y, Wang YS, Chen YS (2009) Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem 19:2710–2714. doi:10.1039/b821416f
Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, Inoue S (1990) Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adryamicin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res 50:1700–1703. doi:10.1016/j.ijpharm.2008.08.011
Yokoyama M, Okano T, Sakurai Y, Suwa D, Kataoka K (1996) Introduction of cisplatin into polymeric micelle. J Control Release 39:351–356. doi:10.1016/0168-3659(95)00165-4
Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, Okano T (1998) Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Release 50:79–92. doi:10.1016/S0168-3659(97)00115-6
Acknowledgements
We thank Anna Gabashvili for the help with cells for confocal microscopy. All authors declare that they have no conflict of interest. This article does not contain any studies with human and animal subjects performed by any of the authors. The study was supported by grant from Russian Scientific Foundation 14-15-00698.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voeikov, R., Abakumova, T., Grinenko, N. et al. Dioxadet-loaded nanogels as a potential formulation for glioblastoma treatment. Journal of Pharmaceutical Investigation 47, 75–83 (2017). https://doi.org/10.1007/s40005-016-0294-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0294-4