Abstract
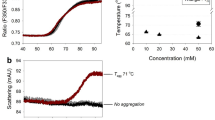
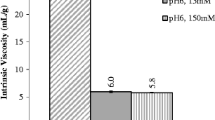
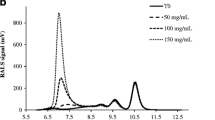
The viscosity of highly concentrated protein solutions was evaluated using lysozyme as model protein. Viscosity profiles of lysozyme were examined with the effect of buffer and pH-value at various concentrations. The viscosity of lysozyme dissolved in water increased continuously with the concentration as the slope of shear stress against shear rate increased with the concentration. In addition, the viscosity of lysozyme was higher in histidine buffer than in acetate buffer at selected pH ranges. The effect of various excipient concentrations was also investigated in means of unfolding transition temperature (T m ), viscosity, hydrodynamic size and zeta potential by using differential scanning calorimetry (DSC), microviscometer and dynamic light scattering (DLS). The selected excipients except surfactants increased the viscosity of protein solution with their concentration. Carbohydrates increased the viscosity relatively higher than amino acids and also they increased the conformational stability (T m ) by enhancing the protein molecule more in compact form. Also amino acids increased the viscosity but decreased the conformational stability since they seemed to be only dispersed in the solution avoiding protein–protein interactions, resulting in a decrease of zeta potential. Consequently, the applied methods—DSC, DLS and microviscometer demonstrated the potential to develop a highly concentrated protein formulation to decrease the high viscosity effect with acceptable conformational stability.





Similar content being viewed by others
References
Chari R, Jerath K, Badkar AV, Kalonia DS (2009) Long- and short-range electrostatic interactions affect the rheology of highly concentrated antibody solutions. Pharm Res 26:2607–2618
Du W, Klibanov AM (2011) Hydrophobic salts markedly diminish viscosity of concentrated protein solutions. Biotechnol Bioeng 108:632–636
Harris RJ, Shire SJ, Winter C (2004) Commercial manufacturing scale formulation and analytical characterization of therapeutic recombinant antibodies. Drug Dev Res 61:137–154
He F, Woods CE, Litowski JR, Roschen LA, Gadgil HS, Razinkov VI, Kerwin BA (2011) Effect of sugar molecules on the viscosity of high concentration monoclonal antibody solutions. Pharm Res 28:1552–1560
Kanai S, Liu J, Patapoff TW, Shire SJ (2008) Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab–Fab interaction that impacts solution viscosity. J Pharm Sci 97:4219–4227
Kim NA, An IB, Lee SY, Park ES, Jeong SH (2012) Optimization of protein solution by a novel experimental design method using thermodynamic properties. Arch Pharm Res 35:1609–1619
Kim NA, Lim DG, Lim JY, Kim KH, Jeong SH (2013) Comprehensive evaluation of etanercept stability in various concentrations with biophysical assessment. Int J Pharm 460:108–118
Kim NA, Lim DG, Lim JY, Kim KH, Jeong SH (2014) Fundamental analysis of recombinant human epidermal growth factor in solution with biophysical methods. Drug Dev Ind Pharm. doi:10.3109/03639045.2013.859152
Liu J, Shire SJ (2005) Reduced-viscosity concentrated protein formulations. US Patent No. 6,875,432
Liu J, Nguyen MD, Andya JD, Shire SJ (2005) Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci 94:1928–1940
Nelson AL, Dhimolea E, Reichert JM (2010) Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 9:767–774
Ohtake S, Kita Y, Arakawa T (2011) Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliv Rev 63:1053–1073
Shire SJ, Shahrokh Z, Liu J (2004) Challenges in the development of high protein concentration formulations. J Pharm Sci 93:1390–1402
Szymanska A, Hornowski T, Slosarek G (2012) Denaturation and aggregation of lysozyme in water-ethanol solution. Acta Biochim Pol 59:317–321
Yadav S, Shire SJ, Kalonia DS (2010) Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci 99:4812–4829
Yadav S, Shire SJ, Kalonia DS (2011) Viscosity analysis of high concentration bovine serum albumin aqueous solutions. Pharm Res 28:1973–1983
Yadav S, Laue TM, Kalonia DS, Singh SN, Shire SJ (2012a) The influence of charge distribution on self-association and viscosity behavior of monoclonal antibody solutions. Mol Pharm 9:791–802
Yadav S, Shire SJ, Kalonia DS (2012b) Viscosity behavior of high-concentration monoclonal antibody solutions: correlation with interaction parameter and electroviscous effects. J Pharm Sci 101:998–1011
Acknowledgments
This article dose not contain any studies with human and animal subjects performed by any of the authors. All authors (N.A. Kim, D.G. Lim, J.Y. Lim, K.H. Kim, W.S. Shim, N. G. Kang, S.H Jeong) declare that they have no conflict of interest. This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012002399) and a grant of the Korean Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea. (Grant No.: A103017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, N.A., Lim, D.G., Lim, J.Y. et al. Evaluation of protein formulation and its viscosity with DSC, DLS, and microviscometer. Journal of Pharmaceutical Investigation 44, 309–316 (2014). https://doi.org/10.1007/s40005-014-0128-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-014-0128-1