Abstract
Stem cell therapy opens a new window in medicine to overcome several diseases that remain incurable. It appears such diseases as cardiovascular disorders, brain injury, multiple sclerosis, urinary system diseases, cartilage lesions and diabetes are curable with stem cell transplantation. However, some questions related to stem cell therapy have remained unanswered. Stem cell imaging allows approval of appropriated strategies such as selection of the type and dose of stem cell, and also mode of cell delivery before being tested in clinical trials. MRI as a non-invasive imaging modality provides proper conditions for this aim. So far, different contrast agents such as superparamagnetic or paramagnetic nanoparticles, ultrasmall superparamagnetic nanoparticles, fluorine, gadolinium and some types of reporter genes have been used for imaging of stem cells. The core subject of these studies is to investigate the survival and differentiation of stem cells, contrast agent’s toxicity and long term following of transplanted cells. The promising results of in vivo and some clinical trial studies may raise hope for clinical stem cells imaging with MRI.


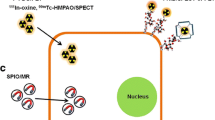
Adopted from Cromer Berman et al. [103], with permission
Similar content being viewed by others
References
Ikehara S. Grand challenges in stem cell treatments. Front Cell Dev Biol. 2013;1:2.
Choumerianou DM, Dimitriou H, Kalmanti M. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008;14:53–60.
Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9.
Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283.
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–67.
Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49.
Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–9.
Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 2013;22:1309–23.
Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–74.
Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272.
Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–78.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22.
Spiriev T, Sandu N, Schaller B. Molecular imaging and tracking stem cells in neurosciences. Methods Mol Biol. 2013;1052:195–201.
Sandu N, Momen-Heravi F, Sadr-Eshkevari P, Schaller B. Molecular imaging for stem cell transplantation in neuroregenerative medicine. Neurodegener Dis. 2012;9:60–7.
McColgan P, Sharma P, Bentley P. Stem cell tracking in human trials: a meta-regression. Stem Cell Rev. 2011;7:1031–40.
Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–6.
Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–44.
Srivastava AK, Kadayakkara DK, Bar-Shir A, Gilad AA, McMahon MT, Bulte JW. Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine. Dis Model Mech. 2015;8:323–36.
Ngen EJ, Artemov D. Advances in monitoring cell-based therapies with magnetic resonance imaging: future perspectives. Int J Mol Sci. 2017;18:E198.
Saito S, Tsugeno M, Koto D, Mori Y, Yoshioka Y, Nohara S, et al. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int J Nanomedicine. 2012;7:5415–21.
Muja N, Bulte JW. Magnetic resonance imaging of cells in experimental disease models. Prog Nucl Magn Reson Spectrosc. 2009;55:61–77.
Jin R, Lin B, Li D, Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol. 2014;18:18–27.
Gutteridge JM, Rowley DA, Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of ‘catalytic’ iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982;206:605–9.
Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333–9.
Barrow M, Taylor A, Murray P, Rosseinsky MJ, Adams DJ. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem Soc Rev. 2015;44:6733–48.
Lee NK, Kim HS, Yoo D, Hwang JW, Choi SJ, Oh W, et al. Magnetic resonance imaging of ferumoxytol-labeled human mesenchymal stem cells in the mouse brain. Stem Cell Rev. 2017;13:127–38.
Brewer KD, Spitler R, Lee KR, Chan AC, Barrozo JC, Wakeel A, et al. Characterization of magneto-endosymbionts as MRI cell labeling and tracking agents. Mol Imaging Biol. 2017. https://doi.org/10.1007/s11307-017-1093-7.
Lee S, Yoon HI, Na JH, Jeon S, Lim S, Koo H, et al. In vivo stem cell tracking with imageable nanoparticles that bind bioorthogonal chemical receptors on the stem cell surface. Biomaterials. 2017;139:12–29.
Liu L, Ho C. Mesenchymal Stem Cell Preparation and Transfection-free Ferumoxytol Labeling for MRI Cell Tracking. Curr Protoc Stem Cell Biol. 2017;43:2B.7.1–14.
Khurana A, Nejadnik H, Chapelin F, Lenkov O, Gawande R, Lee S, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond). 2013;8:1969–83.
Muthiah M, Park IK, Cho CS. Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol Adv. 2013;31:1224–36.
Wang YX, Xuan S, Port M, Idee JM. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr Pharm Des. 2013;19:6575–93.
Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–9.
Heyn C, Bowen CV, Rutt BK, Foster PJ. Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med. 2005;53:312–20.
Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907.
Di Marco M, Sadun C, Port M, Guilbert I, Couvreur P, Dubernet C. Physicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. Int J Nanomedicine. 2007;2:609–22.
Zhao X, Zhao H, Chen Z, Lan M. Ultrasmall superparamagnetic iron oxide nanoparticles for magnetic resonance imaging contrast agent. J Nanosci Nanotechnol. 2014;14:210–20.
Ariza de Schellenberger A, Kratz H, Farr TD, Löwa N, Hauptmann R, Wagner S, et al. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int J Nanomedicine. 2016;11:1517–35.
Morawski AM, Winter PM, Yu X, Fuhrhop RW, Scott MJ, Hockett F, et al. Quantitative “magnetic resonance immunohistochemistry” with ligand-targeted 19F nanoparticles. Magn Reson Med. 2004;52:1255–62.
Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013;26:860–71.
Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One. 2015;10:e0118544.
Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72:1696–701.
Zhong J, Mills PH, Hitchens TK, Ahrens ET. Accelerated fluorine-19 MRI cell tracking using compressed sensing. Magn Reson Med. 2013;69:1683–90.
Gaudet JM, Hamilton AM, Chen Y, Fox MS, Foster PJ. Application of dual 19F and iron cellular MRI agents to track the infiltration of immune cells to the site of a rejected stem cell transplant. Magn Reson Med. 2017;78:713–20.
Tseng CL, Shih IL, Stobinski L, Lin FH. Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials. 2010;31:5427–35.
Ludemann L, Wurm R, Zimmer C. Pharmacokinetic modeling of Gd-DTPA extravasation in brain tumors. Investig Radiol. 2002;37:562–70.
Ngen EJ, Wang L, Kato Y, Krishnamachary B, Zhu W, Gandhi N, et al. Imaging transplanted stem cells in real time using an MRI dual-contrast method. Sci Rep. 2015;5:13628.
Liu Y, He ZJ, Xu B, Wu QZ, Liu G, Zhu H, et al. Evaluation of cell tracking effects for transplanted mesenchymal stem cells with jetPEI/Gd-DTPA complexes in animal models of hemorrhagic spinal cord injury. Brain Res. 2011;1391:24–35.
Guenoun J, Koning GA, Doeswijk G, Bosman L, Wielopolski PA, Krestin GP, et al. Cationic Gd-DTPA liposomes for highly efficient labeling of mesenchymal stem cells and cell tracking with MRI. Cell Transplant. 2012;21:191–205.
Shen J, Cheng LN, Zhong XM, Duan XH, Guo RM, Hong GB. Efficient in vitro labeling rabbit neural stem cell with paramagnetic Gd-DTPA and fluorescent substance. Eur J Radiol. 2010;75:397–405.
Xiao Y, Liu Y, Yang S, Zhang B, Wang T, Jiang D, et al. Sorafenib and gadolinium co-loaded liposomes for drug delivery and MRI-guided HCC treatment. Colloids Surf B Biointerfaces. 2016;141:83–92.
Guo C, Sun L, She W, Li N, Jiang L, Luo K, et al. A dendronized heparin–gadolinium polymer self-assembled into a nanoscale system as a potential magnetic resonance imaging contrast agent. Polym Chem. 2016;7:2531–41.
Murphy SV, Hale A, Reid T, Olson J, Kidiyoor A, Tan J, et al. Use of trimetasphere metallofullerene MRI contrast agent for the non-invasive longitudinal tracking of stem cells in the lung. Methods. 2016;99:99–111.
Gilad AA, Winnard PT Jr, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–90.
Vandsburger MH, Radoul M, Cohen B, Neeman M. MRI reporter genes: applications for imaging of cell survival, proliferation, migration and differentiation. NMR Biomed. 2013;26:872–84.
Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008;10:391–411.
Vande Velde G, Himmelreich U, Neeman M. Reporter gene approaches for mapping cell fate decisions by MRI: promises and pitfalls. Contrast Media Mol Imaging. 2013;8:424–31.
Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008;103:105–13.
Pereira SM, Moss D, Williams SR, Murray P, Taylor A. Overexpression of the MRI reporter genes ferritin and transferrin receptor affect iron homeostasis and produce limited contrast in mesenchymal stem cells. Int J Mol Sci. 2015;16:15481–96.
Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006;56:51–9.
Patrick PS, Rodrigues TB, Kettunen MI, Lyons SK, Neves AA, Brindle KM. Development of Timd2 as a reporter gene for MRI. Patrick PS, Rodrigues TB, Kettunen. 2016;75:1697–707.
Dai HY, He R, Zhang Y, Wu RH, Xiao YY. Adenoviral vector mediated ferritin over-expression in mesenchymal stem cells detected by 7T MRI in vitro. PLoS One. 2017;12:e0185260.
Brewer KD, Spitler R, Lee KR, Chan AC, Barrozo JC, Wakeel A, et al. Characterization of magneto-endosymbionts as MRI cell labeling and tracking agents. Mol Imaging Biol. 2017. https://doi.org/10.1007/s11307-017-1093-7.
Guo R, Li Q, Yang F, Hu X, Jiao J, Guo Y, et al. In vivo MR imaging of dual MRI reporter genes and Deltex-1 gene-modified human mesenchymal stem cells in the treatment of closed penile fracture. Mol Imaging Biol. 2017. https://doi.org/10.1007/s11307-017-1128-0.
Liu M, Wang Y, Li M, Zhang Y, Lan X. Using the tyrosinase gene as a tri-modality reporter gene for monitoring transplanted stem cells in acute myocardial infarction. J Nucl Med. 2017;58:167.
Wu MR, Liu HM, Lu CW, Shen WH, Lin IJ, Liao LW, et al. Organic anion-transporting polypeptide 1B3 as a dual reporter gene for fluorescence and magnetic resonance imaging. FASEB J. 2017. https://doi.org/10.1096/fj.201700767R.
Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601.
Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–3.
Hua P, Wang YY, Liu LB, Liu JL, Liu JY, Yang YQ, et al. In vivo magnetic resonance imaging tracking of transplanted superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells in rats with myocardial infarction. Mol Med Rep. 2015;11:113–20.
Kim YJ, Huh YM, Choe KO, Choi BW, Choi EJ, Jang Y, et al. In vivo magnetic resonance imaging of injected mesenchymal stem cells in rat myocardial infarction; simultaneous cell tracking and left ventricular function measurement. Int J Cardiovasc Imaging. 2009;25:99–109.
Li SH, Lai TY, Sun Z, Han M, Moriyama E, Wilson B, et al. Tracking cardiac engraftment and distribution of implanted bone marrow cells: comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg. 2009;137:1225–33.
Campan M, Lionetti V, Aquaro GD, Forini F, Matteucci M, Vannucci L, et al. Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5-T cardiac MRI in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300:H2238–50.
He G, Zhang H, Wei H, Wang Y, Zhang X, Tang Y, et al. In vivo imaging of bone marrow mesenchymal stem cells transplanted into myocardium using magnetic resonance imaging: a novel method to trace the transplanted cells. Int J Cardiol. 2007;114:4–10.
Sumino Y, Mimata H. Regenerative medicine as a new therapeutic strategy for lower urinary tract dysfunction. Int J Urol. 2013;20:670–5.
Lin CS. Advances in stem cell therapy for the lower urinary tract. World J Stem Cells. 2010;2:1–4.
Adamowicz J, Kloskowski T, Tworkiewicz J, Pokrywczyńska M, Drewa T. Urine is a highly cytotoxic agent: does it influence stem cell therapies in urology? Transplant Proc. 2012;44:1439–41.
Song YS, Ku JH. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol Urodyn. 2007;26:584–93.
Lee HJ, Won JH, Doo SH, Kim JH, Song KY, Lee SJ, et al. Inhibition of collagen deposit in obstructed rat bladder outlet by transplantation of superparamagnetic iron oxide-labeled human mesenchymal stem cells as monitored by molecular magnetic resonance imaging (MRI). Cell Transplant. 2012;21:959–70.
Lee HJ, Doo SW, Kim DH, Cha YJ, Kim JH, Song YS, et al. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Cancer Lett. 2013;335:58–65.
Rivière C, Lecoeur C, Wilhelm C, Péchoux C, Combrisson H, Yiou R, et al. The MRI assessment of intraurethrally—delivered muscle precursor cells using anionic magnetic nanoparticles. Biomaterials. 2009;30:6920–8.
Song YS, Ku JH, Song ES, Kim JH, Jeon JS, Lee KH, et al. Magnetic resonance evaluation of human mesenchymal stem cells in corpus cavernosa of rats and rabbits. Asian J Androl. 2007;9:361–7.
Aghayan HR, Soleimani M, Goodarzi P, Norouzi-Javidan A, Emami-Razavi SH, Larijani B, et al. Magnetic resonance imaging of transplanted stem cell fate in stroke. J Res Med Sci. 2014;19:465–71.
Jendelová P, Herynek V, Urdzikova L, Glogarová K, Kroupová J, Andersson B, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–43.
Syková E, Jendelová P. Magnetic resonance tracking of transplanted stem cells in rat brain and spinal cord. Neurodegener Dis. 2006;3:62–7.
Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med. 2004;10:S42–50.
Sugaya K. Potential use of stem cells in neuroreplacement therapies for neurodegenerative diseases. Int Rev Cytol. 2003;228:1–30.
Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–83.
Zhao JY, Chen G, Gu YP, Cui R, Zhang ZL, Yu ZL, et al. Ultrasmall magnetically engineered Ag2Se quantum dots for instant efficient labeling and whole-body high-resolution multimodal real-time tracking of cell-derived microvesicles. J Am Chem Soc. 2016;138:1893–903.
Kubo T, Baba T, Ikezaki K, Sekiguchi H, Nishino Y, Miyazawa A, et al. Realtime single molecular motion analysis of nicotinic acetylcholine receptor Alpha 7 by diffracted X-Ray tracking method. Biophys J. 2016;110:222a.
Odeleye AOO, Castillo-Avila S, Boon M, Martin H, Coopman K. Development of an optical system for the non-invasive tracking of stem cell growth on microcarriers. Biotechnol Bioeng. 2017;114:2032–42.
Walczak P, Wojtkiewicz J, Nowakowski A, Habich A, Holak P, Xu J, et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J Cereb Blood Flow Metab. 2017;37:2346–58.
Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–8.
Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16:461–6.
Stroh A, Zimmer C, Werner N, Gertz K, Weir K, Kronenberg G, et al. Tracking of systemically administered mononuclear cells in the ischemic brain by high-field magnetic resonance imaging. Neuroimage. 2006;33:886–97.
Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, et al. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS One. 2014;9:e97631.
Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203–5.
Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76.
Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6.
Tang K, Xiao X, Liu D, Shen Y, Chen Y, Wang Y, et al. Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin-induced diabetes in miniature pigs: Real-time tracing with MRI in vivo. Int J Mol Med. 2014;33:1469–76.
Zhang B, Jiang B, Chen Y, Huang H, Xie Q, Kang M, et al. Detection of viability of transplanted beta cells labeled with a novel contrast agent–polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles by magnetic resonance imaging. Contrast Media Mol Imaging. 2012;7:35–44.
Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1–17.
Jing XH, Yang L, Duan XJ, Xie B, Chen W, Li Z, et al. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75:432–8.
Cromer Berman SM, Walczak P, Bulte JW. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:343–55.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal or human experiments carried out for this article.
Rights and permissions
About this article
Cite this article
Yahyapour, R., Farhood, B., Graily, G. et al. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng Regen Med 15, 249–261 (2018). https://doi.org/10.1007/s13770-017-0112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0112-8