Abstract
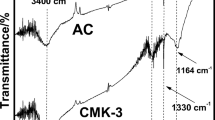
In this investigation, acrylic acid was used as a carbon precursor and SBA-15 as a mesoporous template to synthesize the ordered mesoporous carbons (OMC). Different ratios of SBA-15 to acrylic acid were evaluated to improve the OMCs adsorption capacity. It was found that the optimal ratio of SBA-15 to acrylic acid is 3:1. In the post-treatment study, four methods (NaOH, urea, NH3H2O, and AlCl3) were explored to investigate their influences on resorcinol removal. Results showed the ammonium hydroxide-treated OMC had the highest adsorption capacity of 40.6 mg/g for resorcinol removal. The nano-structures of the OMC and post-treated OMCs were characterized and confirmed. FTIR analysis indicated that the surface functional groups were changed after post-treatments. XRD patterns and TEM images suggested that the ordered structure was well maintained during the post-treatment processes, although the erosion effect was observed.













Similar content being viewed by others
References
Antonio BF (2004) Synthesis of ordered nanoporous carbons of tunable mesopore size by templating SBA-15 silica materials. Micrpor Mesopor Mater 67:273–281
Bazula PA, Lu AH, Nitz JJ, Ferdi S (2008) Surface and pore structure modification of ordered mesoporous carbons via a chemical oxidation approach. Micrpor Mesopor Mater 108:266–275
Cansado IPP, Mourão PAM, Falcão AI, Ribeiro Carrott MML, Carrott PJM (2012) The influence of the activated carbon post-treatment on the phenolic compounds removal. Fuel Process Technol 103:64–70
Fujimori T, Nishimura K, Oshita K, Takeda N, Takaoka M (2014) Influence of the properties of macromolecular carbon on de Novo Synthesis of PCDDs, PCDFs, PCBs, and Chlorobenzenes. Aerosol Air Q Res 14:1131–1141
Gao B, Peng C, Chen GZ, Puma GL (2008) Photo-electro-catalysis enhancement on carbon nanotubes/titanium dioxide (CNTs/TiO2) composite prepared by a novel surfactant wrapping sol–gel method. Appl Catal B 85:17–23
Guo RX, Guo J, Yu FQ, Gang DD (2013) Synthesis and surface functional group modifications of ordered mesoporous carbons for resorcinol removal. Micrpor Mesopor Mater 175:141–146
Kacher J, Landon C, Adam BL, Fullwood D (2009) Bragg’s law diffraction simulations for electron backscatter diffraction analysis. Ultramicroscopy 109(9):1148–1156
Pevida C, Plaza MG, Arias B, Fermoso J, Rubiera F, Pis JJ (2008) Surface modification of activated carbons for CO2 capture. Appl Surf Sci 254:7165–7172
Ramanathan A, Maheswari R, Grady BP, Moore DS, Barich DH, Subramaniam B (2013) Tungsten-incorporated cage-type mesoporous silicate: W-KIT-5. Micrpor Mesopor Mater 175:43–49
Ryong R, Sang HJ, Shinae J (1999) Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J Phys Chem B 103(37):7743–7746
Shafeeyan MS, Daud WM, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143–151
Skar H, Liang YC, Erichsen ES, Anwarder R, Seland JG (2013) Relaxometric properties of gadolinium-grafted mesoporous SBA-15 silica materials with varying pore size. Micrpor Mesopor Mater 75:125–133
Swiatkowski A, Pakula M, Biniak S, Walczyk M (2004) Influence of the surface chemistry of modified activated carbon on its electrochemical behaviour in the presence of lead(II) ions. Carbon 42:3057–3069
Vinke P, Eijk MV, Verbree M, Voskamp AF, VanBekkum H (1994) Modification of the surfaces of a gas activated carbon and a chemically activated carbon with nitric acid, hypochlorite, and ammonia. Carbon 32(4):675–676
Yang H, Zhao D (2005) Synthesis of replica mesostructures by the nanocasting strategy. J Mater Chem 15:1217–1231
Zhang FQ, Meng Y, Gu D, Yan Y, Yu CZ, Tu B, Zhao DY (2005) A facile aqueous route to synthesize highly ordered mesoporous polymers and carbon frameworks with Ia3d bicontinuous cubic structure. J Am Chem Soc 127(39):13508–13509
Acknowledgments
This work was supported by the Louisiana Board of Regents under BORSF (2010-2015) LaSpace and by NASA under award NNX10AI40H.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, H., Shou, W., Ren, C. et al. Preparation and post-treatments of ordered mesoporous carbons (OMC) for resorcinol removal. Int. J. Environ. Sci. Technol. 13, 1505–1514 (2016). https://doi.org/10.1007/s13762-016-0990-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-0990-7