Abstract
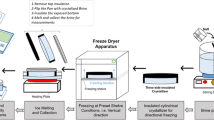
The use of desalination technologies which produce concentrated brines is acutely limited by inadequate waste brine disposal mechanisms such that the brine does not contaminate fresh water resources. The treatment of highly saline brine using freeze desalination technique trade marked as HybridICE™ technology was investigated at pilot scale. The capacity of the HybridICE™ process to generate fresh water by freeze desalination of brine was investigated in this study. Brine samples to feed into the HybridICE process unit were prepared in tanks with volume capacities between 1.0 and 10.0 m3 by dissolving common salt into tape water. The effects of refrigerant temperature, initial brine concentration, energy consumption were evaluated in relation to product ice quality. Feed brine samples were processed in batches in a closed system where it was continuously re-circulated to generate product ice and more concentrated residual small volume of brine stream. The quality of ice produced could be turned into potable water it terms of its low total dissolved salts and conductivity. The salt removal, based on the average chloride concentration in the ice samples, was 96 %. The energy utilization efficiency amounted to an average of ZAR 10.0/m3 water assuming energy cost of ZAR 0.39/kWh. The HybridICE™ technology was shown to be a better option than other desalination technologies currently in use, in terms of energy utilization and cleaner by-products.





Similar content being viewed by others
References
Ahmed M, Shayya WH, Hoey D, Mahendran A, Morris R, Al-Handaly J (2000) Use of evaporation ponds for brine disposal in desalination plants. Desalination 130:155–168
Aider M, De Halleux D (2009) Cryoconcentration technology in the bio-food industry: principles and applications. Food Sci Technol 42(3):679–685
APHA (1998) Standard methods for the examination of water and wastewaters, 20th edn. ANWA, WEF, Washington
Arulampalam GT, Bates C, Khaw LF, McGrath L (1981) Investigation of purification mechanism in a column crystallizer operating under total reflux and continuous conditions. Desalination 36:87–97
Brown GO (2002) Henry Darcy and the making of a law. Water Resour Res 38(7): 11-1–11-12
Buckley CA (2005) Research into the treatment of inorganic brines and concentrates. Water research commission project no. 201, revision 1.021
Buckley CA, Simpson AE, Kerr CA, Schutte CF (1987) The treatment and disposal of waste brine solutions. Desalination 67:431–438
Buhrmann F, van der Waldt M, Hanekom D, Finlayson F (1999) Treatment of industrial wastewater for reuse. Desalination 124:263–269
Dama-Fakir P and Toerien A (2009) Evaporation rates on brine produced during membrane treatment of mine water. In: International Mine Water Conference, Pretoria, South Africa, 19–23 October 2009
Egolf PW, Kauffeld M (2005) From physical properties of ice slurries to industrial ice slurry applications. Refrigeration 28(1):4–12
Flesland O (1995) Freeze concentration by layer crystallization. Dry Technol 12(89):1713–1739
Halde R (1980) Concentration of impurities by progressive freezing. Water Res 14(6):575–580
Johnson WE (1976) State of the art freezing processes, their potential and future. Desalination 19:349–358
Jusoh M, Yunus RM, Abu Hassan MA (2008) Effect of flow rate and coolant temperature on the efficiency of progressive freeze concentration on simulated wastewater. WASET 47:75–78
Jusoh M, Yunus RM, Abu Hassan MA (2009) Performance investigation on a new design for progressive freeze concentration system. J Appl Sci 9(17):3171–3175
Khawaji AD, Kutubkhanah IK, Wie J (2008) Advances in seawater desalination technologies. Desalination 221:47–69
Lewis AE, Nathoo J, Thomsen K, Kramer HJ, Witkamp GJ, Reddy ST, Randall DG (2010) Design of a eutectic freeze crystallization process for multi-component wastewater stream. Chem Eng Res Des 88(9A):1290–1296
Lorain O, Thiebaud P, Badorc E, Aurelle Y (2000) Potential of freezing in wastewater treatment: soluble pollutant applications. Water Res 35(2):541–547
Mahdavi M, Mahvi AH, Nasseri S, Yunesian M (2011) Application of freezing to the desalination of saline water. Arab J Sci Eng 36:1171–1177
Maurer M, Pronk W, Larsen TA (2006) Treatment processes for source-separated urine. Water Res 40(17):3151–3166
Minato W, Shirai Y, Sakashita S (2001) Ice crystallization in a pilot-scale wastewater treatment system. Chem Eng Process 40(3):201–208
Miyawaki O (2001) Analysis and control of ice crystal structure in frozen food and their application to food processing. Food Sci Technol Res 7(1):1–7
Miyawaki O, Liu L, Shirai Y, Sakashita S, Kagitani K (2005) Tubular ice system for scale-up of progressive freeze-concentration. Food Eng 69(1):107–113
Muntinh Y, Mahlaba JS, Pretorius C (2009) Utilising fly ash as a salt sinking media through pasting with industrial brine. In: Proceedings of the congress on engineering (WCE), vol 1, London, July 1–3, pp 1–6
Oosthuizen FS (2000) Application of continuous, calorimetric measuring principle to a technical commercial product: Masters’ thesis, Wirtschaftsakademie Schleswig-Holstein Vocational Academy, Faculty of Economic Engineering, Flensburg
Partyka V (1986) Freezing for wastewater recovery. Met Finish 84(11):55–57
Pronk P, Ferreira CAI, Witkamp GJ (2006) Influence of solute type and concentration on ice scaling in fluidized bed ice crystallizers. Chem Eng Sci 61(13):4354–4362
Qin FGF, Chen XD, Premathilaka S, Free K (2007) Experimental study of wash columns used for separating ice from ice-slurry. Desalination 218(1–3):223–228
Rahman MS, Ahmed M (2007) Freezing–melting process and desalination: review of present status and future prospects. Int J Nucl Desalin 2(3):253–262
Raluy G, Serra L, Uche J (2006) Life cycle assessment of MSF, MED and RO desalination technologies. Energy 31:2361–2372
Randall DG, Nathoo J, Lewis AE (2009) Seeding for selective salt recovery during eutectic freeze crystallization. In: Abstracts of the international mine water conference, Pretoria, pp 639–646, 19–23 Oct 2009
Randall DG, Nathoo J, Lewis AE (2011) A case study for treating a reverse osmosis brine using eutectic freeze crystallization—approaching a zero waste process. Desalination 266(2–3):256–262
Ruemekorf R (1994) Freeze concentration: its application in hazardous wastewater treatment. Environ Sci Pollut Control Ser 7:513–524
Sanchez J, Ruiz Y, Raventos M, Auleda JM, Hernandez E (2010) Progressive freeze concentration for orange juice in a pilot plant falling film. Innov Food Sci Emerg Technol 11:644–651
Schoeman JJ, Steyn A (2000) Investigation into alternative water treatment technologies for the treatment of underground mine water discharged by Grootvlei Proprietary Mines Ltd. into the Blesbokspruit in South Africa. Desalination 133:13–30
Shirai Y, Sugumoto T, Hashimoto M, Nakanishi K, Matsuno R (1987) Mechanisms of ice growth in a batch crystallizer with an external cooler for freeze concentration. Agr Biol Chem Tokyo 51:2359–2366
Shirai Y, Wakisaka M, Miyawaki O, Sakashita S (1998) Conditions of producing an ice layer with high purity for freeze wastewater treatment. Food Eng 38(3):297–308
Shone RDC (1987) The freeze desalination of mine waters. J S Afr Inst Min Metall 87(4):107–112
Shwartz J, Probstein RF (1968) An analysis of counterwashers for freeze distillation desalination. Desalination 4:5–29
Shwartz J, Probstein RF (1969) Experimental study of slurry separators for use in desalination. Desalination 6:239–266
Thijssen HAC (1969) Freeze concentration of food liquids. Food Manuf 44(7):49–54
Thijssen HAC (1975) Apparatus for separation and treatment of solid particles from a liquid suspension. US patent 3,872,009
Vaessen RCJ, Janse BJH, Seckler MM, Witkamp GJ (2003) Evaluation of the performance of a newly developed eutectic freeze crystallizer; scraped cooled wall crystallizer. Inst Chem Eng Trans IchemE Part A 81:1363–1372
Van Der Ham F (1999) Eutectic freeze crystallization. PhD Thesis, Deft University of Technology, The Netherlands
Wakisaka M, Shirai Y, Sakashita, S (2001) Ice crystallization in a pilot-scale freeze wastewater treatment system. Chem Eng Process 40:201–208
Acknowledgments
We thank Tshwane University of Technology, THRIP program of NRF-RSA Grant Number 2010[71807], Rand Water for funding and Aqua-Simon UG for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mtombeni, T., Maree, J.P., Zvinowanda, C.M. et al. Evaluation of the performance of a new freeze desalination technology. Int. J. Environ. Sci. Technol. 10, 545–550 (2013). https://doi.org/10.1007/s13762-013-0182-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0182-7