Abstract
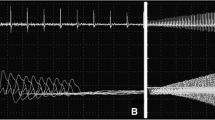
Congenital myasthenic syndromes are rare hereditary disorders caused by mutations associated with proteins of the neuromuscular junction. Abnormal ‘‘gain of function’’ mutations result in prolonged nicotinic acetylcholine receptor channel open state causing a rare subtype of CMS, slow-channel CMS (SCCMS). Mutations in the delta subunit encoding the gene, CHRND, resulting in SCCMS are extremely rare. An important clue to the diagnosis of SCCMS is repetitive CMAP’s. Fluoxetine, usually at high doses, is used to treat SCCMS. The mutation, recently described in one patient, was identified by whole exome sequencing and validated, and its segregation with the disease was ascertained by Sanger sequencing. Here, we describe clinical and genetic findings of an early onset SCCMS patient carrying a very rare missense mutation c.880C > T in CHRND causing a highly conserved leucine to phenylalanine substitution in the M2 domain of CHRND. The patient had no repetitive CMAP. He had a dramatic response to fluoxetine at low–moderate doses (40 mg/day), increasing over months: Being wheelchair bound, he could walk independently after treatment. Rare cases may offer insight into the pathological gating mechanism leading to CMS. SCCMS should be suspected even without a repetitive CMAP. Fluoxetine at relatively low doses can be a very effective treatment.


Similar content being viewed by others
References
Engel AG, Shen XM, Selcen D, Sine SM (2015) Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 14(5):461. https://doi.org/10.1016/S1474-4422(15)00010-1
Abicht A, Dusl M, Gallenmuller C, Guergueltcheva V, Schara U, Della Marina A, Wibbeler E, Almaras S, Mihaylova V, von der Hagen M, Huebner A, Chaouch A, Muller JS, Lochmuller H (2012) Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients. Hum Mutat 33(10):1474–1484. https://doi.org/10.1002/humu.22130
Beeson D (2016) Congenital myasthenic syndromes: recent advances. Curr Opin Neurol 29(5):565–571. https://doi.org/10.1097/WCO.0000000000000370
Engel AG, Ohno K, Milone M, Wang HL, Nakano S, Bouzat C, Pruitt JN, Hutchinson DO, Brengman JM, Bren N, Sieb JP, Sine SM (1996) New mutations in acetylcholine receptor subunit genes reveal heterogeneity in the slow-channel congenital myasthenic syndrome. Hum Mol Genet 5(9):1217–1227. https://doi.org/10.1093/hmg/5.9.1217
Croxen R, Newland C, Beeson D, Oosterhuis H, Chauplannaz G, Vincent A, Newsom-Davis J (1997) Mutations in different functional domains of the human muscle acetylcholine receptor alpha subunit in patients with the slow-channel congenital myasthenic syndrome. Hum Mol Genet 6(5):767–774. https://doi.org/10.1093/hmg/6.5.767
Milone M, Wang HL, Ohno K, Fukudome T, Pruitt JN, Bren N, Sine SM, Engel AG (1997) Slow-channel myasthenic syndrome caused by enhanced activation, desensitization, and agonist binding affinity attributable to mutation in the M2 domain of the acetylcholine receptor alpha subunit. J Neurosci 17(15):5651–5665
Shen XM, Okuno T, Milone M, Otsuka K, Takahashi K, Komaki H, Giles E, Ohno K, Engel AG (2016) Mutations causing slow-channel myasthenia reveal that a valine ring in the channel pore of muscle AChR is optimized for stabilizing channel gating. Hum Mutat 37(10):1051–1059. https://doi.org/10.1002/humu.23043
Tan JZ, Man Y, Xiao F (2016) A missense mutation in epsilon-subunit of acetylcholine receptor causing autosomal dominant slow-channel congenital myasthenic syndrome in a chinese family. Chin Med J (Engl) 129(21):2596–2602. https://doi.org/10.4103/0366-6999.192780
Angelini C, Lispi L, Salvoro C, Mostacciuolo ML, Vazza G (2019) Clinical and genetic characterization of an Italian family with slow-channel syndrome. Neurol Sci 40(3):503–507. https://doi.org/10.1007/s10072-018-3645-2
Gomez CM, Maselli RA, Vohra BP, Navedo M, Stiles JR, Charnet P, Schott K, Rojas L, Keesey J, Verity A, Wollmann RW, Lasalde-Dominicci J (2002) Novel delta subunit mutation in slow-channel syndrome causes severe weakness by novel mechanisms. Ann Neurol 51(1):102–112. https://doi.org/10.1002/ana.10077[pii]
Chaouch A, Muller JS, Guergueltcheva V, Dusl M, Schara U, Rakocevic-Stojanovic V, Lindberg C, Scola RH, Werneck LC, Colomer J, Nascimento A, Vilchez JJ, Muelas N, Argov Z, Abicht A, Lochmuller H (2012) A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol 259(3):474–481. https://doi.org/10.1007/s00415-011-6204-9
Durmus H, Shen XM, Serdaroglu-Oflazer P, Kara B, Parman-Gulsen Y, Ozdemir C, Brengman J, Deymeer F, Engel AG (2018) Congenital myasthenic syndromes in Turkey: Clinical clues and prognosis with long term follow-up. Neuromuscul Disord 28(4):315–322. https://doi.org/10.1016/j.nmd.2017.11.013S0960-8966(17)31232-4 [pii]
Harper CM, Engel AG (1998) Quinidine sulfate therapy for the slow-channel congenital myasthenic syndrome. Ann Neurol 43(4):480–484. https://doi.org/10.1002/ana.410430411
Harper CM, Fukodome T, Engel AG (2003) Treatment of slow-channel congenital myasthenic syndrome with fluoxetine. Neurology 60(10):1710–1713
Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346(4):967–989. https://doi.org/10.1016/j.jmb.2004.12.031
Unwin N, Fujiyoshi Y (2012) Gating movement of acetylcholine receptor caught by plunge-freezing. J Mol Biol 422(5):617–634. https://doi.org/10.1016/j.jmb.2012.07.010S0022-2836(12)00577-3 [pii]
Engel AG, Sine SM (2005) Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol 5(3):308–321. https://doi.org/10.1016/j.coph.2004.12.007
Sayle RA, Milner-White EJ (1995) RASMOL: biomolecular graphics for all. Trends Biochem Sci 20(9):374
Shen XM, Milone M, Wang HL, Banwell B, Selcen D, Sine SM, Engel AG (2019) Slow-channel myasthenia due to novel mutation in M2 domain of AChR delta subunit. Ann Clin Transl Neurol 6(10):2066–2078. https://doi.org/10.1002/acn3.50902
Colomer J, Muller JS, Vernet A, Nascimento A, Pons M, Gonzalez V, Abicht A, Lochmuller H (2006) Long-term improvement of slow-channel congenital myasthenic syndrome with fluoxetine. Neuromuscul Disord 16(5):329–333. https://doi.org/10.1016/j.nmd.2006.02.009S0960-8966(06)00073-3 [pii]
Pitt M (2008) Neurophysiological strategies for the diagnosis of disorders of the neuromuscular junction in children. Dev Med Child Neurol 50(5):328–333. https://doi.org/10.1111/j.1469-8749.2008.02038.x
Mihaylova V, Muller JS, Vilchez JJ, Salih MA, Kabiraj MM, D'Amico A, Bertini E, Wolfle J, Schreiner F, Kurlemann G, Rasic VM, Siskova D, Colomer J, Herczegfalvi A, Fabriciova K, Weschke B, Scola R, Hoellen F, Schara U, Abicht A, Lochmuller H (2008) Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain 131(Pt 3):747–759. https://doi.org/10.1093/brain/awm325
Lorenzoni PJ, Scola RH, Gervini BL, Kay CS, Werneck LC (2009) Electrophysiological study in synaptic congenital myasthenic syndrome: end-plate acetylcholinesterase deficiency. Arq Neuropsiquiatr 67(2B):502–504 S0004-282X2009000300024 [pii]
Brownlow S, Webster R, Croxen R, Brydson M, Neville B, Lin JP, Vincent A, Newsom-Davis J, Beeson D (2001) Acetylcholine receptor delta subunit mutations underlie a fast-channel myasthenic syndrome and arthrogryposis multiplex congenita. J Clin Invest 108(1):125–130. https://doi.org/10.1172/JCI12935
Shen XM, Ohno K, Fukudome T, Tsujino A, Brengman JM, De Vivo DC, Packer RJ, Engel AG (2002) Congenital myasthenic syndrome caused by low-expressor fast-channel AChR delta subunit mutation. Neurology 59(12):1881–1888
Shen XM, Fukuda T, Ohno K, Sine SM, Engel AG (2008) Congenital myasthenia-related AChR delta subunit mutation interferes with intersubunit communication essential for channel gating. J Clin Invest 118(5):1867–1876. https://doi.org/10.1172/JCI34527
Muller JS, Baumeister SK, Schara U, Cossins J, Krause S, von der Hagen M, Huebner A, Webster R, Beeson D, Lochmuller H, Abicht A (2006) CHRND mutation causes a congenital myasthenic syndrome by impairing co-clustering of the acetylcholine receptor with rapsyn. Brain 129(Pt 10):2784–2793. https://doi.org/10.1093/brain/awl188
Miyazawa A, Fujiyoshi Y, Unwin N (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423(6943):949–955. https://doi.org/10.1038/nature01748
Webster R, Maxwell S, Spearman H, Tai K, Beckstein O, Sansom M, Beeson D (2012) A novel congenital myasthenic syndrome due to decreased acetylcholine receptor ion-channel conductance. Brain 135(Pt 4):1070–1080. https://doi.org/10.1093/brain/aws016
Beckstein O, Sansom MS (2006) A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Phys Biol 3(2):147–159. https://doi.org/10.1088/1478-3975/3/2/007S1478-3975(06)18065-6 [pii]
Unwin N (1995) Acetylcholine receptor channel imaged in the open state. Nature 373(6509):37–43. https://doi.org/10.1038/373037a0
Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376(6540):514–516. https://doi.org/10.1038/376514a0
Gomez CM, Gammack JT (1995) A leucine-to-phenylalanine substitution in the acetylcholine receptor ion channel in a family with the slow-channel syndrome. Neurology 45(5):982–985
Funding
Supported by Scientific Research Project Coordination Unit of Istanbul University. Project Number: 33507.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to disclose.
Ethical statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durmus, H., Sticht, H., Ceylaner, S. et al. Rare slow channel congenital myasthenic syndromes without repetitive compound muscle action potential and dramatic response to low dose fluoxetine. Acta Neurol Belg 121, 1755–1760 (2021). https://doi.org/10.1007/s13760-020-01505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-020-01505-0