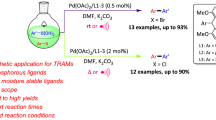
Abstract
An organic solvent free and efficient heterogeneous synthesis for bridging heteroaryl halides and 4-fluorophenylboronic acid was studied in aqueous media according to the Suzuki cross-coupling protocol. High yields of heteroaryl-aryl fluorides were successfully obtained with: chloro-/bromo-substituted pyridine, thiophene, indole, and inzole in neat water using palladium phosphinous acid complexes (POPd)/tetrabutylammonium bromide (TBAB) as co-catalysts. A possible mechanism for the heterogeneous coupling reaction is proposed and discussed according to the function of the TBAB interphases. The notable properties of the reported method are highly co-catalytic activity, hetero-atom tolerance, simple separating procedure and little environmental disposal impact.



Similar content being viewed by others
References
M. Sharif, A. Maalik, S. Reimann, H. Feist, J. Iqbal, T. Patonay, A. Villinger, P. Langer, J. Fluorine Chem. 146, 19 (2013)
M. Zhu, W.J. Fu, G.L. Zou, C. Xu, Z.Q. Wang, J. Fluorine Chem. 163, 23 (2014)
K. Müller, ChemBioChem 5, 637 (2004)
M. Shimizu, T. Hiyama, Angew. Chem. Int. Ed. 44, 214 (2005)
T. Furuya, J.E. Klein, T. Ritter, Synthesis 11, 1804 (2010)
M. Döbele, S. Vanderheiden, N. Jung, S. Bräse, Angew. Chem. Int. Ed. 49, 5986 (2010)
L. Keyes, A.D. Sun, J.A. Love, Eur. J. Org. Chem. 3985 (2011)
H.J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem 5, 637 (2004)
S.M. Ametamey, M. Honer, P.A. Schubiger, Chem. Rev. 108, 1501 (2008)
W.B. Yi, J.J. Ma, J.Q. Jiang, C. Cai, W. Zhang, J. Fluorine Chem. 157, 84 (2014)
T. Nöel, T.J. Maimone, S.L. Buchwald, Angew. Chem. Int. Ed. 50, 8900 (2011)
T.J. Maimone, P.J. Milner, T. Kinzel, Y. Zhang, M.K. Takase, S.L. Buchwald, J. Am. Chem. Soc. 133, 18106 (2011)
Z.Q. Yua, Y.W. Lv, C.M. Yu, W.K. Su, Tetrahedron Lett. 54, 1261 (2013)
K.K. Laali, V.J. Gettwert, J. Fluorine Chem. 107, 31 (2001)
M.J. Brown, V. Gouverneur, Angew. Chem. Int. Ed. 48, 8610 (2009)
J.W. Graskemper, B.J. Wang, L.L. Qin, K.D. Neumann, S.G. DiMagno, Org. Lett. 13, 3158 (2011)
G. Sandford, J. Fluorine Chem. 128, 90 (2007)
N. Miyaura, A. Suzuki, Chem. Rev. 95, 2457 (1995)
A. Suzuki, J. Organomet. Chem. 576, 147 (1999)
T. Fujita, N. Suzuki, T. Ichitsuka, J. Ichikawa, J. Fluorine Chem. 155, 97 (2013)
Q.P. Ding, H.F. Ji, D. Wang, Y.Q. Lin, W.H. Yu, Y.Y. Peng, J. Organomet. Chem. 711, 62 (2012)
B. Li, C.P. Wang, G. Chen, Z.Q. Zhang, J. Environ. Sci. 6, 1083 (2013)
G.Y. Li, J. Org. Chem. 67, 3643 (2002)
C. Wolf, K. Ekoue-Kovi, Eur. J. Org. Chem. 1917 (2006)
S.P. Khanapure, D.S. Garvey, Tetrahedron Lett. 45, 5283 (2004)
T. Okazaki, K.K. Laali, S.D. Bunge, S.K. Adas, Eur. J. Org. Chem. 1630 (2014)
C. Peng, G.B. Yan, Y. Wang, Y.B. Jiang, Y. Zhang, J.B. Wan, Synthesis 24, 4154 (2010)
R.B. De Vasher, L.R. Moore, K.H. Shaughnessy, J. Org. Chem. 69, 7919 (2004)
A. Arcadi, G. Cerichelli, M. Chiarini, M. Correa, D. Zorzan, Eur. J. Org. Chem. 4080 (2003)
D. Badone, M. Baroni, R.I. Cardamone, A. Lelmini, U. Guzzi, J. Org. Chem. 62, 7170 (1997)
J.C. Bussolari, D.C. Rehborn, Org. Lett. 1, 965 (1999)
R.B. Bedford, M.E. Blake, C.P. Butts, D. Holder, Chem. Commun. 466 (2003)
Acknowledgments
We are grateful for financial support from the Program for Liaoning Excellent Talents in University (No.LR2014009), (No.L2015258) and the kind donation of Pd-catalysts from Combiphos Catalysts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Zhang, Z. POPd/TBAB co-catalyzed Suzuki cross-coupling reaction of heteroaryl chlorides/bromides with 4-fluorophenylboronic acid in water. J IRAN CHEM SOC 13, 637–644 (2016). https://doi.org/10.1007/s13738-015-0775-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0775-9