Abstract
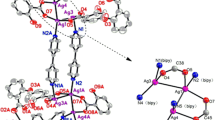
Two silver(I) supramolecular polymers, [Ag(8-HqH)(8-Hq)] n (1) and {[Ag(8-HqH)2]NO3} n (2), (8-HqH = 8-hydroxyquinoline), have been synthesized and characterized by X-ray powder diffraction, IR, 1H-NMR and 13C-NMR spectroscopies. These supramolecular polymers were used as new precursors for preparation of silver nanostructures. The effect of thermolysis temperature on preparation of silver nanoparticles from these precursors was investigated. The morphology of silver nanostructure is strongly dependent on thermolysis temperature. With increase in temperature, the tendency of silver nanoparticles to agglomerate was increased.




Similar content being viewed by others
References
S. Kitagawa, R. Kitaura, S.-I. Noro, Angew. Chem. Int. Ed. 43, 2334 (2004)
R.J. Kuppler, D.J. Timmons, Q.-R. Fang, J.-R. Li, T.A. Makal, M.D. Young, D. Yuan, D. Zhao, W. Zhuang, H.-C. Zhou, Coord. Chem. Rev. 253, 3042 (2009)
K. Akhbari, A. Morsali, Dalton Trans. 42, 4786 (2013)
Z.-J. Li, S.K. Khani, K. Akhbari, A. Morsali, P. Retailleau, Microporous Mesoporous Mater. 199, 93 (2014)
K.W. Hellmann, L.H. Gade, R. Fleischer, D. Stalke, Chem. Commun. 527 (1997)
J.A. Zerowski, C.T. Seto, G.M. Whitesides, J. Am. Chem. Soc. 114, 5473 (1992)
J.-M. Lehn, M. Mascal, A. DeCian, J. Fischer, J. Chem. Soc. Chem. Commun. 479 (1990)
Y.-L. Chang, M.-A. West, F.W. Fowler, J.W. Lauher, J. Am. Chem. Soc. 115, 5991 (1993)
E.A.H. Griffith, E.L. Amma, J. Am. Chem. Soc. 96, 743 (1974)
H.G. Smith, R.E. Rundle, J. Am. Chem. Soc. 80, 5075 (1958)
H. Schmidbaur, W. Bublak, B. Huber, G. Reber, G. Muller, Angew. Chem. Int. Ed. Engl. 25, 1089 (1986)
G.L. Ning, L.P. Wu, K. Sugimoto, M. Munakata, T. Kuroda-Sowa, M. Mackawa, J. Chem. Soc. Dalton Trans. 2529 (1999)
M. Munakata, L.P. Wu, T. Kuroda-Sowa, M. Maekawa, Y. Suenaga, G.L. Ning, T. Kojima, J. Am. Chem. Soc. 120, 8610 (1998)
R. Bashiri, K. Akhbari, A. Morsali, M. Zeller, J. Organomet. Chem. 693, 1903 (2008)
K. Akhbari, A. Morsali, S. Rafiei, M. Zeller, J. Organomet. Chem. 693, 257 (2008)
K. Akhbari, A. Morsali, M. Zeller, J. Organomet. Chem. 692, 3788 (2007)
K. Akhbari, A. Morsali, L.-G. Zhu, J. Mol. Struct. 891, 132 (2008)
M. Jansen, Angew. Chem. Int. Ed. 26, 1098 (1987)
C.-S. Liu, P.-Q. Chen, E.-C. Yang, J.-L. Tian, X.-H. Bu, Z.-M. Li, H.-W. Sun, Z. Lin, Inorg. Chem. 45, 5812 (2006). (and references cited therein)
C.-S. Liu, P.-Q. Chen, Z. Chang, J.-J. Wang, L.-F. Yan, H.-W. Sun, X.-H. Bu, Z. Lin, Z.-M. Li, S.R. Batten, Inorg. Chem. Commun. 11, 159 (2008)
C. Santini, C. Pettinari, G. Gioia Lobbia, R. Spagna, M. Pellei, F. Vallorani, Inorg. Chim. Acta 285, 81 (1999)
H. Liang, Q. Tang, K. Yu, S. Li, J. Ke, Mater. Lett. 61, 1020 (2007)
M.Y. Masoomi, A. Morsali, Coord. Chem. Rev. 256, 2921 (2012)
R. Das, P. Pachfule, R. Banerjee, P. Poddar, Nanoscale 4, 591 (2012)
M. Moeinian, K. Akhbari, J. Solid State Chem. 225, 459 (2015)
F. Shahangi Shirazi, K. Akhbari, RSC Adv. 5, 50778 (2015)
F. Shahangi Shirazi, K. Akhbari, Inorg. Chem. Acta. 436, 1 (2015)
K. Akhbari, A. Morsali, Z. Anorg, Allg. Chem. 638, 692 (2012)
K. Akhbari, A. Morsali, Cryst. Eng. Commun. 12, 3394 (2010)
K. Akhbari, M. Hemmati, A. Morsali, J. Inorg. Organomet. Polym. 21, 352 (2011)
K. Akhbari, A. Morsali, Inorg. Chem. Acta. 363, 1435 (2010)
S. Hojaghani, K. Akhbari, M. Hossaini Sadr, A. Morsali, Inorg. Chem. Commun. 44, 1 (2014)
K. Akhbari, A. Morsali, P. Retailleau, Polyhedron 29, 3304 (2010)
R. Bashiri, K. Akhbari, A. Morsali, Inorg. Chem. Acta. 362, 1035 (2009)
H. Wu, X.-W. Dong, H.-Y. Liu, J.-F. Ma, Acta Crystallogr. Sect. E 62, m281 (2006)
K. Akhbari, A. Morsali, Inorg. Chem. 52, 2787 (2013)
C.F. Macrae, P.R. Edgington, P. McCabe, E. Pidcock, G.P. Shields, R. Taylor, M. Towler, J. van de Streek, J. Appl. Cryst. 39, 453 (2006)
P. Pyykkö, Chem. Rev. 97, 597 (1997)
Acknowledgments
The authors would like to acknowledge the financial support of the University of Tehran for this research under grant number 01/1/389845 and support of this investigation by Tarbiat Modares University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
13738_2015_724_MOESM1_ESM.doc
Supplementary material 1 (DOC 1230 kb) Supplementary material Electronic Supplementary Information (ESI) including experimental data, IR, 1H-NMR, 13C-NMR spectra and XRD patterns are available
Rights and permissions
About this article
Cite this article
Akhbari, K., Bahman, N.B., Morsali, A. et al. The effect of thermolysis temperatures of two silver(I) supramolecular polymers on the formation of silver nanostructures. J IRAN CHEM SOC 13, 165–169 (2016). https://doi.org/10.1007/s13738-015-0724-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0724-7