Abstract
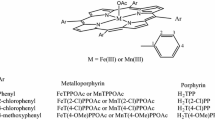
In this study, the catalytic activity of meso-tetra(n-propyl)porphyrinatomanganese(III) acetate, MnT(n-pr)(OAc) in oxidation of olefins and sulfides with tetra-n-butylammonium Oxone (TBAO), tetra-n-butylammonium periodate (TBAP), aqueous hydrogen peroxide, sodium periodate and Oxone in the presence of imidazole (ImH) has been studied. The comparison of catalytic performance of MnT(n-pr)P(OAc) and MnTPP(OAc) in oxidation of olefins with TBAP shows that while the latter is four times more efficient than the former, the extent of oxidative degradation of the former is ca. 3.5 times greater than the latter. The use of excess amount of styrene resulted in only a ca. 10 % increase in the catalyst stability, suggesting a mainly intramolecular mechanism for the catalyst degradation. On the other hand, in the case of TBAO, the oxidative degradation of the former is four times greater than the latter, but the catalytic performance of the latter for the oxidation of cyclohexene was only ca. 2 times larger than the former. This observation shows that the decreased catalytic performance of MnT(n-pr)P(OAc) relative to MnTPP(OAc) is essentially due to the high degree of degradation of the former. Due to the high degree of catalyst degradation, oxidation of olefins with periodate and Oxone in the presence of the two manganese porphyrins in aqueous solution (or with hydrogen peroxide in dichloromethane) gave little or no product. Oxidation of sulfides with TBAO and TBAP in the presence of MnT(n-pr)P(OAc) showed a conversion of ca. 15 % for the catalytic oxidation of sulfides to sulfones.




Similar content being viewed by others
References
B. Meunier, Chem. Rev. 92, 1411 (1992)
D. Mansuy, Pure Appl. Chem. 66, 737 (1994)
J.L. McLain, J. Lee, J.T. Groves, B. Meunier, R.A. Sheldon, in Biomimetic oxidations catalyzed by transition metal complexes, ed. by B. Meunier, Chapters 3, 4 and 14 (Imperial College Press, London, 2000)
S.D. Black, M.J. Coon, in Cytochrome P-450, structure, mechanism and biochemistry, ed. by P. R. O. de Montellano (Plenum Press, New York, 1986), p. 161
D. Mohajer, S. Tangestaninejad, J. Chem. Soc. Chem. Commun. 1993, 240 (1993)
D. Mohajer, R. Tayebee, H. Goudarziafshar, J. Chem. Res. (S) 1999, 168 (1999)
D. Mohajer, G. Karimipour, M. Bagherzadeh, New J. Chem. 28, 740 (2004)
S. Zakavi, F. Heidarizadi, S. Rayati, Inorg. Chem. Commun. 14, 1010 (2011)
S. Zakavi, A.G. Mojarrad, S. Rayati, J. Mol. Catal. A: Chem. 363–364, 153 (2012)
S. Zakavi, T. Mokary Yazdeli, J. Mol. Catal. A Chem. 367, 108 (2013)
T. Wijesekera, J.E. Lyons, P.E. Ellis, M.V. Bhinde, US Patent 5,770,728 (1998)
S. Zakavi, A.S. Ashtiani, S. Rayati, Polyhedron 29, 1492 (2010)
S. Zakavi, S. Talebzadeh, S. Rayati, Polyhedron 31, 368 (2012)
I.D. Cunningham, T.N. Danks, J.N. Hay, I. Hamerton, S. Gunathilagan, C. Janczak, J. Mol. Catal. A Chem. 185, 25 (2002)
S. Neya, N. Funasaki, J. Heterocyclic Chem. 34, 689 (1997)
A.D. Adler, F.R. Longo, J.D. Finarelli, J. Goldmacher, J. Assour, L. Korsakoff, J. Org. Chem. 32, 476 (1967)
A.D. Adler, F.R. Longo, F. Kampas, J. Kim, J. Inorg. Nucl. Chem. 32, 2443 (1970)
A.C. Serra, E.C. Marçalo, AMd’A Rocha Gonsalves, J. Mol. Catal. A Chem. 215, 17 (2004)
L. Mahmoudi, D. Mohajer, R. Kissner, W.H. Koppenol, Dalton Trans. 40, 8695 (2011)
D. Mohajer, M. Abbasi, Eur. J. Inorg. Chem. 2008, 3218 (2008)
D. Mohajer, A. Rezaeifard, Tetrahedron Lett. 43, 1881 (2002)
S. Rayati, S. Zakavi, H. Kalantari, J. Porphyrin Phthalocyanines 15, 131 (2011)
S. Rayati, S. Zakavi, P. Jafarzadeh, O. Sadeghi, M.M. Amini, J. Porphyrin Phthalocyanines 16, 260 (2012)
S. Zakavi, R. Omidyan, L. Ebrahimi, F. Heidarizadi, Inorg. Chem. Commun. 14, 1827 (2011)
R. Koerner, M.M. Olmstead, A. Ozarowski, S.L. Phillips, P.M. Van Calcar, K. Winkler, A.L. Balch, J. Am. Chem. Soc. 120, 1274 (1998)
M. Gouterman, J. Mol. Spectrosc. 6, 138 (1961)
C. Bruckner, E.D. Sternberg, J.K. MacAlpine, S.J. Rettig, D. Dolphin, J. Am. Chem. Soc. 121, 2609 (1999)
B. Cheng, O.Q. Munro, H.M. Marques, R. Scheidt, J. Am. Chem. Soc. 119, 10732 (1997)
N. Meot-Ner, A.D. Adler, J. Am. Chem. Soc. 97, 5107 (1975)
D. Mohajer, S. Zakavi, S. Rayati, M. Zahedi, N. Safari, H.R. Khavasi, S. Shahbazian, New J. Chem. 28, 1600 (2004)
Acknowledgments
Financial Support of this work by the Institute for Advanced Studies in Basic Sciences (IASBS) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zakavi, S., Fathi, M. Oxidation of olefins and sulfides with different oxidants catalyzed by meso-tetra(n-propyl)porphyrinatomanganese(III) acetate: comparison with meso-tetra(phenyl)porphyrinatomanganese(III) acetate. J IRAN CHEM SOC 11, 1667–1674 (2014). https://doi.org/10.1007/s13738-014-0439-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0439-1