Abstract
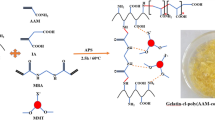
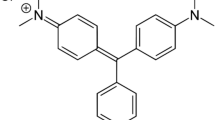
Gum xanthan/psyllium-based nanocomposite was prepared by microwave-assisted synthetic method for the removal of toxic Malachite green (MG) dye from aqueous solutions. The nanocomposite was prepared by in situ incorporation of the K2Zn3[Fe(CN)6]·9H2O nanoparticles into the semi-IPN matrix in the presence of ammonium persulphate and glutaraldehyde as initiator-crosslinker system. Liquid uptake efficacy of the hybrid superabsorbent was enhanced through the optimization of different reaction conditions, including APS = 0.027 mol L−1; glutaraldehyde = 0.053 × 10−3 mol L−1; solvent = 8.0 mL; acrylic acid = 10.928 mol L−1; pH 7.0; reaction time = 60 s and microwave power = 100 % and its thermal behavior was evaluated using TGA-DTG-DTA technique. Candidate nanocomposite was characterized by FTIR, SEM, XRD and UV–visible spectroscopic methods. Various optimized parameters for the efficient removal (83 %) of the Malachite green were adsorbent dose of 800 mg, 14 mg L−1 initial dye concentration and contact time of 28 h. Further, Langmuir and Freundlich adsorption isotherms showed good applicability in adsorption process of MG onto the nanocomposite with maximum adsorption efficiency of 3.21 mg g−1. However, for Freundlich isotherm, R 2 was around 0.9947 and value of 1/n was less than 1 for the synthesized nanocomposite which indicated that the Freundlich isotherm was more favorable than Langmuir isotherm model along with its usability for wide range of dye concentrations. The nanocomposite was found to be a potential product for dye removal from waste water and could prove to be a boon for textile sector.









Similar content being viewed by others
References
Bonetto LR, Ferrarini F, De Marco C, Crespo JS, Guegan R, Giovanela M (2015) Removal of methyl violet 2B dye from aqueous solution using a magnetic composite as an adsorbent. J Water Process Eng 6:11–20
Bulut E, Ozacar M, Sengil I (2008) Adsorption of Malachite green onto bentonite: equilibrium and kinetic studies and process design. Micropor Mesopor Mat 115:234–246
Kushwaha AK, Gupta N, Chattopadhyaya MC (2014) Removal of cationic methylene blue and Malachite green dyes from aqueous solution by waste materials of Daucus carota. J Saudi Chem Soc 18:200–207
Garg VK, Kumar R, Gupta R (2004) Removal of Malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria. Dyes Pigments 62:1–10
Mittal H, Jindal R, Kaith BS, Maity A, Ray SS (2015) Flocculation and adsorption properties of biodegradable gum-ghatti-grafted poly(acrylamide-co-methacrylic acid) hydrogels. Carbohyd Polym 115:617–628
Geetha P, Latha MS, Koshy M (2015) Biosorption of Malachite green dye from aqueous solution by calcium alginate nanoparticles: equilibrium study. J Mol Liq 212:723–730
Khankrua R, Pivsa-Art S, Hiroyuki H, Suttiruengwong S (2013) Thermal and mechanical properties of biodegradable polyester/silica nanocomposites. Energ Procedia 34:705–713
Jancar J, Douglas JF, Starr FW, Kumar SK, Cassagnau P, Lesser AJ, Sternstein SS, Buehler MJ (2010) Current issues in research on structure-property relationships in polymer nanocomposites. Polymer 51:3321–3343
Oladipo AA, Gazi M, Yilmaz E (2015) Single and binary adsorption of azo and anthraquinone dyes by chitosan-based hydrogel: selectivity factor and Box-Behnken process design. Chem Eng Res Des 104:264–279
Zhang YR, Wang SQ, Shen SL, Zhao BX (2013) A novel water treatment magnetic nanomaterial for removal of anionic and cationic dyes under severe condition. Chem Eng J 233:258–264
Shirsath SR, Patil AP, Bhanvase BA, Sonawane SH (2015) Ultrasonically prepared poly(acrylamide)-kaolin composite hydrogel for removal of crystal violet dye from wastewater. J Environ Chem Eng 3:1152–1162
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Ali SR, Chandra P, Latwal M, Jain SK, Bansal VK, Singh SP (2011) Synthesis of nickel hexacyanoferrate nanoparticles and their potential as heterogeneous catalysts for the solvent-free oxidation of benzyl alcohol. Chinese J Catal 32:1844–1849
Sharma K, Kumar V, Kaith BS, Kumar V, Som S, Kalia S, Swart HC (2015) Synthesis, characterization and water retention study of biodegradable gum ghatti-poly(acrylic acid-aniline) hydrogels. Polym Degrad Stab 111:20–31
Saruchi Kaith BS, Jindal R, Kumar V (2015) Biodegradation of gum tragacanth acrylic acid based hydrogel and its impact on soil fertility. Polym Degrad Stab 115:24–31
Kumar N, Mittal H, Parashar V, Ray SS, Ngila JC (2016) Efficient removal of rhodamine 6G dye from aqueous solution using nickel sulphide incorporated polyacrylamide grafted gum karaya bionanocomposite hydrogel. RSC Adv 6:21929–21939
Zhang Q, Zhang T, He T, Chen L (2014) Removal of crystal violet by clay/PNIPAm nanocomposite hydrogels with various clay contents. Appl Clay Sci 90:1–5
Khattri SD, Singh MK (2009) Removal of Malachite green from dye wastewater using neem sawdust by adsorption. J Hazard Mater 167:1089–1094
Sharma K, Kaith BS, Kumar V, Kalia S, Kumar V, Swart HC (2014) Water retention and dye adsorption behavior of Gg-cl-poly(acrylic acid-aniline) based conductive hydrogels. Geoderma 232–234:45–55
Sharma K, Kaith BS, Kumar V, Kumar V, Kalia S, Kapur BK, Swart HC (2014) A comparative study of the effect of Ni9+ and Au8+ ion beams on the properties of poly (methacrylic acid) grafted gum ghatti films. Radiat Phys Chem 97:253–261
Sharma K, Kumar V, Kaith BS, Kumar V, Som S, Kalia S, Swart HC (2014) A study of the biodegradation behaviour of poly(methacrylic acid/aniline)-grafted gum ghatti by a soil burial method. RSC Adv 4:25637–25649
Idris MN, Ahmad ZA, Ahmad MA (2011) Adsorption equilibrium of Malachite green dye onto rubber seed coat based activated carbon. Int J Basic Appl Sci 11:38–43
Banerjee C, Ghosh S, Sen G, Mishra S, Shukla P, Bandopadhyay R (2013) Study of algal biomass harvesting using cation in guar gum from the natural plant source as flocculant. Carbohyd Polym 92:675–681
Jassal V, Shanker U, Kaith BS, Shankar S (2015) Green synthesis of potassium zinc hexacyanoferrate nanocubes and their potential application in photocatalytic degradation of organic dyes. RSC Adv 5:26141–26149
Mahdavinia GR, Aghaie H, Sheykhloie H, Vardini MT, Etemadi H (2013) Synthesis of CarAlg/MMt nanocomposite hydrogels and adsorption of cationic crystal violet. Carbohyd Polym 98:358–365
Joseph F, Agrawal YK, Rawtani D (2013) Behavior of Malachite green with different adsorption matrices. Front Life Sci 7:99–111
Ahmad MA, Alrozi R (2011) Removal of Malachite green dye from aqueous solution using rambutan peel-based activated carbon: equilibrium, kinetic and thermodynamic studies. Chem Eng J 171:510–516
Kao S, Lin Y, Chin K, Hu C, Leung M, Ho K (2014) High contrast and low-driving voltage electrochromic device containing triphenylaminedendritic polymer and zinc hexacyanoferrate. Sol Energ Mat Sol Cells 125:261–267
Ayad MM, El-Nasr AA (2010) Adsorption of cationic dye (methylene blue) from water using polyaniline nanotubes base. J Phys Chem C 114:14377–14383
Baek M, Ijagbemi CO, Se-Jin O, Kim D (2010) Removal of Malachite Green from aqueous solution using degreased coffee bean. J Hazard Mater 176:820–828
Khan AA, Ahmad R, Khan A, Mondal PK (2013) Preparation of unsaturated polyester Ce(IV) phosphate by plastic waste bottles and its application for removal of Malachite green dye from water samples. Arab J Chem 6:361–368
Zhou C, Wu Q, Lei T, Negulescu II (2014) Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem Eng J 251:17–24
Acknowledgments
One of the authors is extremely thankful to MHRD for providing fellowship for carrying out this research work. The author is also thankful to IUAC, New Delhi, Instrumentation Center, IIT, Ropar, for characterization of the samples, and DST-FIST New Delhi for financial assistance in acquiring the FTIR and UV–Vis spectrophotometers at NIT Jalandhar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaith, B.S., Sukriti, Sharma, J. et al. Microwave-assisted green synthesis of hybrid nanocomposite: removal of Malachite green from waste water. Iran Polym J 25, 787–797 (2016). https://doi.org/10.1007/s13726-016-0467-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-016-0467-z