Abstract
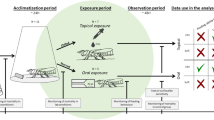
The toxicity of three insecticides frequently used in Neotropical tomato cultivation (abamectin, deltamethrin, and methamidophos) was estimated on foragers of the Neotropical stingless bee Melipona quadrifasciata (Lep.) and the honey bee Apis mellifera (L.). Our results showed that the susceptibility varied significantly with the type of exposure (ingestion, topical, or contact), and there were significant differences between species. While M. quadrifasciata was usually more susceptible to insecticides (except for abamectin) in realistic exposures (via ingestion and contact) than A. mellifera, the former was less susceptible than A. mellifera to topically applied insecticides, a less realistic means of insecticide exposure. These findings challenge the common extrapolation of toxicity bioassays with A. mellifera to all (native) bee pollinators. Such equivocated extrapolation may compromise the significant services provided by native bees in Neotropical ecosystems.
Similar content being viewed by others
References
Antonini, Y., Costa, R.G., Martins, R.P. (2006) Floral preferences of a neotropical stingless bee, Melipona quadrifasciata Lepeletier (Apidae: Meliponina) in an urban forest fragment. Braz. J. Biol. 66, 463–471
Becher, M.A., Osborne, J.L., Thorbek, P., Kennedy, P.J., Grimm, V. (2013) Towards a systems approach for understanding honeybee decline: a stocktaking and synthesis of existing models. J. Appl. Ecol. 50(4), 868–880
Biddinger, D.J., Robertson, J., Mullin, C., Frazier, J., Ashcraft, S., Rajotte, E.G., Joshi, N.K., Vaughn, M. (2013) Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS ONE 8(9), e72587
Biesmeijer, J.C., Roberts, S.P.M., Reemer, M., Ohlemüller, R., Edwards, M., et al. (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313(5785), 351–354
Bispo dos Santos, S., Roselino, A., Hrncir, M., Bego, L. (2009) Pollination of tomatoes by the stingless bee Melipona quadrifasciata and the honey bee Apis mellifera (Hymenoptera: Apidae). Genet. Mol. Res. 8, 751–757
Brittain, C., Potts, S.G. (2011) The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 12, 321–331
Cameron, S.A., Lozier, J.D., Strange, J.P., Koch, J.B., Cordes, N., Solter, L.F., Griswold, T.L. (2011) Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U. S. A. 108(2), 662–667
Cornman, R., Tarpy, D., Chen, Y., Jeffreys, L., Lopez, D., Pettis, J.S., vanEngelsdorp, D., Evans, D. (2012) Pathogen webs in collapsing honey bee colonies. PLoS ONE 7(8), e43562
Cresswell, J.E., Page, C.J., Uygun, M.B., Holmbergh, M., Li, Y., et al. (2012) Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115(6), 365–371
Cruz, D., Freitas, B., Silva, L., EMS, S., Bomfim, I. (2005) Pollination efficiency of the stingless bee Melipona subnitida on greenhouse sweet pepper. Pesq. Agrop. Brasileira 40(12), 1197–1201
Decourtye, A., Henry, M., Desneux, N. (2013) Overhaul pesticide testing on bees. Nature 497, 188
Del Sarto, M.C.L., Peruquetti, R.C., Campos, L.A.O. (2005) Evaluation of the Neotropical stingless bee Melipona quadrifasciata (Hymenoptera: Apidae) as pollinator of greenhouse tomatoes. J. Econ. Entomol. 98(2), 260–266
Devillers, J., Pham-Delègue, M., Decourtye, A., Budzinski, H., Cluzeau, S., et al. (2003) Modeling the acute toxicity of pesticides to Apis mellifera. Bull. Insectol. 56(1), 103–109
EPA (2014) ECOTOX database (http://cfpub.epa.gov/ecotox/), United States Environmental Protection Agency (Accessed on 20th Jan 2014)
Felton, J., Oomen, P., Stevenson, J. (1986) Toxicity and Hazard of pesticides to honeybees: harmonization of test methods. Bee World 67, 114–124
Freitas, B.M., Paxton, R.J. (1998) A comparison of two pollinators: the introduced honey bee Apis mellifera and an indigenous bee Centris tarsata on cashew Anacardium occidentale in its native range of NE Brazil. J. Appl. Ecol. 35(1), 109–121
Freitas, B.M., Imperatriz-Fonseca, V.L.C., Medina, L.M., Kleinert, A.D.M.P., Galetto, L., Nates-Parra, G., Quezada-Euan, J.J.G. (2009) Diversity, threats and conservation of native bees in the Neotropics. Apidologie 40(3), 332–346
Garibaldi, L.A., Steffan-Dewenter, I., Winfree, R., Aizen, M.A., Bommarco, R., et al. (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339(6127), 1608–1611
Gill, R., Ramos-Rodriguez, O., Raine, N. (2012) Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–109
Hardstone, Scott, J. (2010) Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag. Sci. 66(66), 1171–1180
IUCN (2013) IUCN red list of endangered species. In: International Union for Conservation of Nature and Natural Resources (Ed.), pp. http://www.iucnredlist.org/ [Accessed 2 Oct 2013]
Jha, S., Kremen, C. (2013) Resource diversity and landscape-level homogeneity drive native bee foraging. Proc. Natl. Acad. Sci. U. S. A. 110(2), 555–558
Johnson, R.M., Ellis, M.D., Mullin, C.A., Frazier, M. (2010) Pesticides and honey bee toxicity - USA. Apidologie 41(3), 312–331
Kevan, P.G. (1999) Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric. Ecosyst. Environ. 74(1–3), 373–393
Kremen, C., Williams, N.M., Thorp, R.W. (2002) Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. U. S. A. 99(26), 16812–16816
Krupke, C., Hunt, G., Eitzer, B., Andino, G., Given, K. (2012) Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7(1), e29268
Lautenbach, S., Seppelt, R., Liebscher, J., Dormann, C.F. (2012) Spatial and temporal trends of global pollination benefit. PLoS ONE 7(4), e35954
Liu, Z., Williamson, M.S., Lansdell, S.J., Denholm, I., Han, Z., Millar, N.S. (2005) A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc. Natl. Acad. Sci. U. S. A. 102(24), 8420–8425
Lourenço, C., Carvalho, S.M., Malaspina, O., Nocelli, R.C.F. (2012) Oral toxicity of fipronil insecticide against the stingless bee Melipona scutellaris (Latreille, 1811). Bull. Environ. Contam. Toxicol. 89(4), 921–924
Luz, C., Fernandes-Salomão, T., Lage, L., Resende, H., Tavares, M. et al. (2011) Pollen sources for Melipona capixaba Moure & Camargo: an endangered Brazilian stingless bee. Psyche 2011(Article ID 107303), 1–7
Maeta, Y., Kitamura, T. (1981) Pollinating efficiency by Osmia cornifrons (Radoszkowski) in relation to required number of nesting bees for economic fruit production. Honeybee Sci 2, 65–72 (in Japanese)
MAPA (2013) AGROFIT: Sistema de Agrotóxicos Fitossanitários - MAPA/CGAF/DFIA/DAS. In: Ministério da Agricultura Pecuária e Abastecimento (Ed.), Brasilia, Brazil, pp. http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons [Accessed on October 2nd, 2013]
Moraes, S.S., Bautista, A., Viana, B. (2000) Avaliação da toxicidade aguda (DL50 e CL50) de insecticidas para Scaptotrigona tubida (Smith) (Hymenoptera: Apidae): via de contacto. Ann. Soc. Entomol. Bras 29, 31–37
Morandin, L.A., Winston, M.L., Franklin, M.T., Abbott, V.A. (2005) Lethal and sub-lethal effects of spinosad on bumble bees (Bombus impatiens Cresson). Pest Manag. Sci. 61(7), 619–626
Mullin, C., Frazier, M., Frazier, J., Ashcraft, S., Simonds, R., vanEngelsdorp, D., Pettis, J.S. (2010) High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5(3), e9754
Nunes-Silva, P., Hrncir, M., Silva, C.I., Roldão, Y., Imperatriz-Fonseca, V. (2013) Stingless bees, Melipona fasciculata, as efficient pollinators of eggplant (Solanum melongena) in greenhouses. Apidologie 44(5), 537–546
OECD (1998a) Guidelines for the testing of chemicals. Number 213, Honeybees, acute oral toxicity test. In: OECD Environmental Health and Safety Division (ed.), Paris
OECD (1998b) Guidelines for the testing of chemicals. Number 214. Honeybees, acute contact toxicity test. In: OECD Environmental Health and Safety Division (Ed.)
Osborne, J.L. (2012) Bumblebees and pesticides. Nature 491, 43–45
Pettis, J., Lichtenberg, E., Andree, M., Stitzinger, J., Rose, R., vanEngelsdorp, D. (2013) Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8(7), e70182
Potts, S.G., Biesmeijer, J.C., Kremen, C., Neumann, P., Schweiger, O., Kunin, W.E. (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25(6), 345–353
Resende, H., Barros, F., Campos, L.A.O., Fernandes-Salomão, T. (2008) Visitação de orquídea por Melipona capixaba Moure & Camargo (Hymenoptera: Apidae), abelha ameaçada de extinção. Neotropical Entomol. 37, 609–611
Robertson, J.L., Preisler, H.K. (1992) Pesticide bioassays with arthropods. CRC, Boca Raton, FL, USA
SAS Institute (2008) SAS/STAT user’s guide. SAS Institute Inc, Cary
Silveira, A.V.T., Antoniosi-Filho, N.R. (2013) Proposta de alternativas menos tóxicas para ingredientes ativos de agrotóxicos no mercado brasileiro. Pestic. R. Ecotoxicol. Meio Amb. 23, 11–24
Thompson, Wilkins, S., Battersby, A., Waite, R., Wilkinson, D. (2007) Modelling long-term effects of IGRs on honey bee colonies. Pest Manag. Sci. 63, 1081–1084
Tirado, R., Simon, G., Johnston, P., A. Greenpeace International, The Netherlands. (2013) Bees in declilne – a review of factors that put pollinators and agriculture in Europe at risk. In: Greenpeace International (ed.), Greenpeace Research Laboratories Technical Report (Review) 01/2013, Amsterdam
Tomé, H., Martins, G., Lima, M., Campos, L.A.O., Guedes, R. (2012) Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PLoS ONE 7(6), e38406
Valdovinos-Núñez, G.R., Quezada-Euán, J.J.G., Ancona-Xiu, P., Moo-Valle, H., Carmona, A., Sanchez, E.R. (2009) Comparative toxicity of pesticides to stingless bees (Hymenoptera: Apidae: Meliponini). J. Econ. Entomol. 102(5), 1737–1742
vanEngelsdorp, D., Meixner, M.D. (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103, S80–S95
Winfree, R., Williams, N.M., Dushoff, J., Kremen, C. (2007) Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 10(11), 1105–1113
Acknowledgments
This work was supported by grants from the Arthur Bernardes Foundation (FUNARBE), Minas Gerais State Foundation for Research Aid (FAPEMIG), the National Council of Scientific and Technological Development (CNPq), and the CAPES Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Monique Gauthier
Différence de susceptibilité aux insecticides entre l’abeille sans aiguillon néotropicale Melipona quadrifasciata et l’abeille domestique Apis mellifera
Exposition à un insecticide / toxicité aigüe / pollinisateur / abeille sauvage
Unterschiedliche Empfindlichkeit gegenüber Insektiziden bei der neotropischen stachellosen Biene Melipona quadrifasciata und der Honigbiene Apis mellifera
Insektizidbelastung / akute Toxizität / Vibrationsbestäubung / wildlebende Bienen
Rights and permissions
About this article
Cite this article
Del Sarto, M.C.L., Oliveira, E.E., Guedes, R.N.C. et al. Differential insecticide susceptibility of the Neotropical stingless bee Melipona quadrifasciata and the honey bee Apis mellifera . Apidologie 45, 626–636 (2014). https://doi.org/10.1007/s13592-014-0281-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-014-0281-6