Abstract
Background
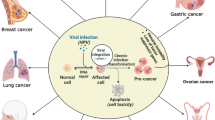
Human papillomavirus (HPV) is a non-enveloped, double-stranded DNA virus. HPV infection occurs through sexual route, and the virus infects mucosal and cutaneous epithelial cells. Inside the cell, the viral DNA replicates extrachromosomally. HPV is the major cause of cervical cancer worldwide. HPVs infecting mucosal epithelial cells are sub-grouped into low-risk or high-risk by virtue of them causing benign warts or cancer, respectively. The early oncoproteins of HPV, namely E4, E5, E6 and E7, are known to contribute to tumorigenesis. The roles and functions of HPV E6 and E7 have been thoroughly studied over the years. However, limited studies have been done on E5 regarding its intracellular functions.
Conclusions
This review attempts to discuss the positive role of HPV16 E5 in the form of therapeutic target for cervical cancer, as well as its role in modulation of several intracellular signalling pathways leading to transformed phenotype of the host cell.



Similar content being viewed by others
References
M.S. Longworth, L.A. Laimins, Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 68, 362–372 (2004)
H. Jo, J.W. Kim, Implications of HPV infection in uterine cervical cancer. Cancer Ther. 3, 419–434 (2005)
T.T. Li, L.N. Zhao, Z.G. Liu, Y. Han, D.M. Fan, Regulation of apoptosis by the papillomavirus E6 oncogene. World J. Gastroenterol 11, 931–937 (2005)
M. Sur, K. Cooper, The role of the human papilloma virus in esophageal cancer. Pathology 30, 348–354 (1998)
G.M. Clifford, J.S. Smith, M. Plummer, N. Munoz, S. Franceschi, Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer 88, 63–73 (2003)
S. Smith, L. Lindsay, B. Hoots, J. Keys, S. Franceschi, R. Winer, G.M. Clifford, Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int. J. Cancer 121, 621–632 (2007)
H. zur Hausen, Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 9, 405–411 (1999)
H. zur Hausen, Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2, 342–350 (2002)
E.M. de Villiers, K. Gunst, H. Stein, H. Scherubl, Esophageal squamous cell cancer in patients with head and neck cancer: prevalence of human papillomavirus DNA sequences. Int. J. Cancer 109, 253–258 (2004)
N.A. Khan, A. Castillo, C. Koriyama, Y. Kijima, Y. Umekita, Y. Ohi, M. Higashi, Y. Sagara, H. Yoshinaka, T. Tsuji, S. Natsugoe, T. Douchi, Y. Eizuru, S. Akiba, Human papillomavirus detected in female breast carcinomas in Japan. Br. J. Cancer 99, 408–414 (2008)
P.M. Reidy, H.H. Dedo, R. Rabah, J.B. Field, R.H. Mathog, L. Gregoire, W.D. Lancaster, Integration of human papillomavirus type 11 in recurrent respiratory papilloma-associated cancer. Laryngoscope 114, 1906–1909 (2004)
R.L. Ferris, I. Martinez, N. Sirianni, J. Wang, A. Lopez-Albaitero, S.M. Gollin, J.T. Johnson, S. Khan, Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur. J. Cancer 41, 807–815 (2005)
C. Fakhry, M.L. Gillison, Clinical implications of human papillomavirus in head and neck cancers. J. Clin. Oncol. 24, 2606–2611 (2006)
S. Motoyama, C.A. Ladines-Llave, S. Luis Villanueva, T. Maruo, The role of human papilloma virus in the molecular biology of cervical carcinogenesis. Kobe J. Med. Sci. 50, 9–19 (2004)
K. Middleton, W. Peh, S. Southern, H. Griffin, K. Sotlar, T. Nakahara, A. El-Sherif, L. Morris, R. Seth, M. Hibma, D. Jenkins, P. Lambert, N. Coleman, J. Doorbar, Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J. Virol. 77, 10186–10201 (2003)
J. Doorbar, The papillomavirus life cycle. J. Clin. Virol. 32, S7–S15 (2005)
P. Finzer, A. Aguilar-Lemarroy, F. Rosl, The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 188, 15–24 (2002)
E.C. Thorland, S.L. Myers, B.S. Gostout, D.I. Smith, Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene 22, 1225–1237 (2003)
M.J. Ferber, E.C. Thorland, A.A. Brink, A.K. Rapp, L.A. Phillips, R. McGovern, B.S. Gostout, T.H. Cheung, T.K. Chung, W.Y. Fu, D.I. Smith, Preferential integration of human papillomavirus type 18 near the c-myc locus in cervical carcinoma. Oncogene 22, 7233–7242 (2003)
N.C. Popescu, J.A. DiPaolo, Integration of human papillomavirus 16 DNA and genomic rearrangements in immortalized human keratinocyte lines. Cancer Res. 50, 1316–1323 (1990)
C.C. Ragin, S.C. Reshmi, S.M. Gollin, Mapping and analysis of HPV16 integration sites in a head and neck cancer cell line. Int. J. Cancer 110, 701–709 (2004)
M.J. Ferber, D.P. Montoya, C. Yu, I. Aderca, A. McGee, E.C. Thorland, D.M. Nagorney, B.S. Gostout, L.J. Burgart, L. Boix, J. Bruix, B.J. McMahon, T.H. Cheung, T.K. Chung, Y.F. Wong, D.I. Smith, L.R. Roberts, Oncogene 22, 3813–3820 (2003)
N. Wentzensen, S. Vinokurova, M. von Knebel Doeberitz, Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 64, 3878–3884 (2004)
N. Hafner, C. Driesch, M. Gajda, L. Jansen, R. Kirchmayr, I.B. Runnebaum, M. Durst, Integration of the HPV16 genome does not invariably result in high levels of viral oncogene transcripts. Oncogene 27, 1610–1617 (2008)
B.A. Werness, A.J. Levine, P.M. Howley, Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248, 76–79 (1990)
N. Dyson, P.M. Howley, K. Munger, E. Harlow, The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243, 934–937 (1989)
N. Ganguly, S.P. Parihar, Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J. Biosci. 34, 113–123 (2009)
D. DiMaio, D. Mattoon, Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene 20, 7866–7873 (2001)
E. Schwarz, U.K. Freese, L. Gissmann, W. Mayer, B. Roggenbuck, A. Stremlau, H. zur Hausen, Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314, 111–114 (1985)
M. Conrad, V.J. Bubb, R. Schlegel, The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J. Virol. 67, 6170–6178 (1993)
M.I. Rodriguez, M.E. Finbow, A. Alonso, Binding of human papillomavirus 16 E5 to the 16 kDa subunit c (proteolipid) of the vacuolar H+ -ATPase can be dissociated from the E5-mediated epidermal growth factor receptor overactivation. Oncogene 19, 3727–3732 (2000)
C. Oetke, E. Auvinen, M. Pawlita, A. Alonso, Human papillomavirus type 16 E5 protein localizes to the Golgi apparatus but does not grossly affect cellular glycosylation. Arch. Virol. 145, 2183–2191 (2000)
P. Thomsen, B. van Deurs, B. Norrild, L. Kayser, The HPV16 E5 oncogene inhibits endocytic trafficking. Oncogene 19, 6023–6032 (2000)
E.S. Hwang, T. Nottoli, D. Dimaio, The HPV16 E5 protein: expression, detection, and stable complex formation with transmembrane proteins in COS cells. Virology 211, 227–233 (1995)
C. Lewis, M.F. Baro, M. Marques, M. Gruner, A. Alonso, I.G. Bravo, The first hydrophobic region of the HPV16 E5 protein determines protein cellular location and facilitates anchorage-independent growth. Virol. J. 5, 30 (2008)
P. Leechanachai, L. Banks, F. Moreau, G. Matlashewski, The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene 7, 19–25 (1992)
S.W. Straight, P.M. Hinkle, R.J. Jewers, D.J. McCance, The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J. Virol. 67, 4521–4532 (1993)
V. Bouvard, G. Matlashewski, Z.M. Gu, A. Storey, L. Banks, The human papillomavirus type 16 E5 gene cooperates with the E7 gene to stimulate proliferation of primary cells and increases viral gene expression. Virology 203, 73–80 (1994)
G.F. Valle, L. Banks, The human papillomavirus (HPV)-6 and HPV-16 E5 proteins co-operate with HPV-16 E7 in the transformation of primary rodent cells. J. Gen. Virol. 76, 1239–1245 (1995)
M.C. Stoppler, S.W. Straight, G. Tsao, R. Schlegel, D.J. McCance, The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology 223, 251–254 (1996)
I. Oelze, J. Kartenbeck, K. Crusius, A. Alonso, Human papillomavirus type 16 E5 protein affects cell-cell communication in an epithelial cell line. J. Virol. 69, 4489–4494 (1995)
J.L. Chang, Y.P. Tsao, D.W. Liu, S.J. Huang, W.H. Lee, S.L. Chen, The expression of HPV-16 E5 protein in squamous neoplastic changes in the uterine cervix. J. Biomed. Sci. 8, 206–213 (2001)
S.M. Genther Williams, G.L. Disbrow, R. Schlegel, D. Lee, D.W. Threadgill, P.F. Lambert, Requirement of epidermal growth factor receptor for hyperplasia induced by E5, a high-risk human papillomavirus oncogene. Cancer Res. 65, 6534–6542 (2005)
J.P. Maufort, S.M. Williams, H.C. Pitot, P.F. Lambert, Human papillomavirus 16 E5 oncogene contributes to two stages of skin carcinogenesis. Cancer Res. 67, 6106–6112 (2007)
J.P. Maufort, A. Shai, H.C. Pitot, P.F. Lambert, A role for HPV16 E5 in cervical carcinogenesis. Cancer Res. 70, 2924–2931 (2010)
L. Hu, K. Plafker, V. Vorozhko, R.E. Zuna, M.H. Hanigan, G.J. Gorbsky, S.M. Plafker, P.C. Angeletti, B.P. Ceresa, Human papillomavirus 16 E5 induces bi-nucleated cell formation by cell-cell fusion. Virology 384, 125–134 (2009)
L. Hu, B.P. Ceresa, Characterization of the plasma membrane localization and orientation of HPV16 E5 for cell-cell fusion. Virology 393, 135–143 (2009)
L. Hu, T.A. Potapova, S. Li, S. Rankin, G.J. Gorbsky, P.C. Angeletti, B.P. Ceresa, Expression of HPV16 E5 produces enlarged nuclei and polyploidy through endoreplication. Virology 405, 342–351 (2010)
J.L. Brandsma, Y. Sun, P.M. Lizardi, D.P. Tuck, D. Zelterman, G.K. Haines 3rd, M. Martel, M. Harigopal, K. Schofield, M. Neapolitano, Distinct human papillomavirus type 16 methylomes in cervical cells at different stages of premalignancy. Virology 389, 100–107 (2009)
V. Badal, L.S. Chuang, E.H. Tan, S. Badal, L.L. Villa, C.M. Wheeler, B.F. Li, H.U. Bernard, CpG methylation of human papillomavirus type 16 DNA in cervical cancer cell lines and in clinical specimens: genomic hypomethylation correlates with carcinogenic progression. J. Virol. 77, 6227–6234 (2003)
C.J. Piyathilake, M. Macaluso, R.D. Alvarez, M. Chen, S. Badiga, J.C. Edberg, E.E. Partridge, G.L. Johanning, A higher degree of methylation of the HPV 16 E6 gene is associated with a lower likelihood of being diagnosed with cervical intraepithelial neoplasia. Cancer 117, 957–963 (2011)
R. Jaenisch, A. Bird, Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254 (2003)
A.P. Feinberg, B. Vogelstein, Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 301, 89–92 (1983)
M.A. Gama-Sosa, V.A. Slagel, R.W. Trewyn, R. Oxenhandler, K.C. Kuo, C.W. Gehrke, M. Ehrlich, The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 11, 6883–6894 (1983)
P.A. Jones, DNA methylation and cancer. Oncogene 21, 5358–5360 (2002)
A.J. Dannenberg, S.M. Lippman, J.R. Mann, K. Subbaramaiah, R.N. DuBois, Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J. Clin. Oncol. 23, 254–266 (2005)
D. Pim, M. Collins, L. Banks, Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene 7, 27–32 (1992)
B. Zhang, A. Srirangam, D.A. Potter, A. Roman, HPV16 E5 protein disrupts the c-Cbl-EGFR interaction and EGFR ubiquitination in human foreskin keratinocytes. Oncogene 24, 2585–2588 (2005)
K. Crusius, E. Auvinen, A. Alonso, Enhancement of EGF- and PMA-mediated MAP kinase activation in cells expressing the human papillomavirus type 16 E5 protein. Oncogene 15, 1437–1444 (1997)
K. Crusius, I. Rodriguez, A. Alonso, The human papillomavirus type 16 E5 protein modulates ERK1/2 and p38 MAP kinase activation by an EGFR-independent process in stressed human keratinocytes. Virus Genes 20, 65–69 (2000)
K. Crusius, E. Auvinen, B. Steuer, H. Gaissert, A. Alonso, The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp. Cell Res. 241, 76–83 (1998)
A. Pedroza-Saavedra, E.W. Lam, F. Esquivel-Guadarrama, L. Gutierrez-Xicotencatl, The human papillomavirus type 16 E5 oncoprotein synergizes with EGF-receptor signaling to enhance cell cycle progression and the down-regulation of p27(Kip1). Virology 400, 44–52 (2010)
Y.P. Tsao, L.Y. Li, T.C. Tsai, S.L. Chen, Human papillomavirus type 11 and 16 E5 represses p21(WafI/SdiI/CipI) gene expression in fibroblasts and keratinocytes. J. Virol. 70, 7535–7539 (1996)
C.E. Eberhart, R.J. Coffey, A. Radhika, F.M. Giardiello, S. Ferrenbach, R.N. DuBois, Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107, 1183–1188 (1994)
A. Ristimaki, N. Honkanen, H. Jankala, P. Sipponen, M. Harkonen, Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 57, 1276–1280 (1997)
M. Kondo, H. Yamamoto, H. Nagano, J. Okami, Y. Ito, J. Shimizu, H. Eguchi, A. Miyamoto, K. Dono, K. Umeshita, N. Matsuura, K. Wakasa, S. Nakamori, M. Sakon, M. Monden, Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 5, 4005–4012 (1999)
S. Kulkarni, J.S. Rader, F. Zhang, H. Liapis, A.T. Koki, J.L. Masferrer, K. Subbaramaiah, A.J. Dannenberg, Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 7, 429–434 (2001)
K. Kim, Y.T. Jeon, I.A. Park, J.W. Kim, N.H. Park, S.B. Kang, H.P. Lee, Y.S. Song, Cyclooxygenase-2 expression in cervical intraepithelial neoplasia. Ann. N. Y. Acad. Sci. 1171, 111–115 (2009)
J.M. Oh, S.H. Kim, Y.I. Lee, M. Seo, S.Y. Kim, Y.S. Song, W.H. Kim, Y.S. Juhnn, Human papillomavirus E5 protein induces expression of the EP4 subtype of prostaglandin E2 receptor in cyclic AMP response element-dependent pathways in cervical cancer cells. Carcinogenesis 30, 141–149 (2009)
S.H. Kim, Y.S. Juhnn, S. Kang, S.W. Park, M.W. Sung, Y.J. Bang, Y.S. Song, Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell Mol. Life Sci. 63, 930–938 (2006)
S.L. Chen, S.T. Lin, T.C. Tsai, W.C. Hsiao, Y.P. Tsao, ErbB4 (JM-b/CYT-1)-induced expression and phosphorylation of c-jun is abrogated by human papillomavirus type 16 E5 oncoprotein. Oncogene 26, 42–53 (2007)
A. Venuti, D. Salani, F. Poggiali, V. Manni, A. Bagnato, The E5 oncoprotein of human papillomavirus type 16 enhances endothelin-1-induced keratinocyte growth. Virology 248, 1–5 (1998)
K. Kabsch, A. Alonso, The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J. Virol. 76, 12162–12172 (2002)
M. Scheffner, B.A. Werness, J.M. Huibregtse, A.J. Levine, P.M. Howley, The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes degradation of p53. Cell 63, 1129–1136 (1990)
J.M. Oh, S.H. Kim, E.A. Cho, Y.S. Song, W.H. Kim, Y.S. Juhnn, Human papillomavirus type 16 E5 protein inhibits hydrogen-peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis 31, 402–410 (2010)
B. Zhang, D.F. Spandau, A. Roman, E5 protein of human papillomavirus type 16 protects human foreskin keratinocytes from UV B-irradiation-induced apoptosis. J. Virol. 76, 220–231 (2002)
E. Auvinen, A. Alonso, P. Auvinen, Human paillomavirus type 16 E5 protein colocalizes with antiapoptotic Bcl-2 protein. Arch. Virol. 149, 1745–1759 (2004)
E. Krawczyk, J.A. Hanover, R. Schlegel, F.A. Suprynowicz, Karyopherin beta3: a new cellular target for the HPV-16 E5 oncoprotein. Biochem. Biophys. Res. Commun. 371, 684–688 (2008)
B. Zhang, P. Li, E. Wang, Z. Brahmi, K.W. Dunn, J.S. Blum, A. Roman, The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-gamma. Virology 310, 100–108 (2003)
S. Miura, K. Kawana, D.J. Schust, T. Fujii, T. Yokoyama, Y. Iwasawa, T. Nagamatsu, K. Adachi, A. Tomio, K. Tomio, S. Kojima, T. Yasugi, S. Kozuma, Y. Taketani, CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J. Virol. 84, 11614–11623 (2010)
M. Gruener, I.G. Bravo, F. Momburg, A. Alonso, P. Tomakidi, The E5 protein of the human papillomavirus type 16 down-regulates HLA-I surface expression in calnexin-expressing but not in calnexin-deficient cells. Virol. J. 4, 116 (2007)
D. Greco, N. Kivi, K. Qian, S.K. Leivonen, P. Auvinen, E. Auvinen, Human papillomavirus 16 E5 modulates the expression of host micro-RNAs. PLoS One 6, (2011)
D.W. Liu, Y.P. Tsao, C.H. Hsieh, J.T. Hsieh, J.T. Kung, C.L. Chiang, S.J. Huang, S.L. Chen, Induction of CD8 T cells by vaccination with recombinant adenovirus expressing human papillomavirus type 16 E5 gene reduces tumor growth. J. Virol. 74, 9083–9089 (2000)
N. Vigneron, B.J. Van den Eynde, Insights into the processing of MHC class I ligands gained from the study of human tumor epitopes. Cell Mol. Life Sci. 68, 1503–1520 (2011)
S.P. Cullen, M. Brunet, S.J. Martin, Granzymes in cancer and immunity. Cell Death Differ. 17, 616–623 (2010)
Y.F. Chen, C.W. Lin, Y.P. Tsao, S.L. Chen, Cytotoxic-T-lymphocyte human papillomavirus type 16 E5 peptide with CpG-oligodeoxynucleotide can eliminate tumor growth in C57BL/6 mice. J. Virol. 78, 1333–1343 (2004)
N. Sima, W. Wang, D. Kong, D. Deng, Q. Xu, J. Zhou, G. Xu, L. Meng, Y. Lu, S. Wang, D. Ma, RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulation of Rb and p53. Apoptosis 13, 273–281 (2008)
M.K. Kim, H.S. Kim, S.H. Kim, J.M. Oh, J.Y. Han, J.M. Lim, Y.S. Juhnn, Y.S. Song, Human papillomavirus type 16 E5 oncoprotein as a new target for cervical cancer treatment. Biochem. Pharmacol. 80, 1930–1935 (2010)
K.S. Anderson, J. Wong, G. D’Souza, A.B. Riemer, J. Lorch, R. Haddad, S.I. Pai, J. Longtine, M. McClean, J. LaBaer, K.T. Kelsey, M. Posner, Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. Br. J. Cancer 104, 1896–1905 (2011)
P. Gao, J. Zheng, High-risk HPV induced cell fusion: a critical initiating event in the early stage of HPV associated cervical cancer. Virol. J. 7, 238 (2010)
K.C. Foy, Z. Liu, G. Phillips, M. Miller, P.T. Kaumaya, Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J. Biol. Chem. 286, 13626–13637 (2011)
Acknowledgements
The funding from Cancer Research UK is duly acknowledged.