Abstract
Background
Somatostatin (SS) acts as a universal endocrine off-switch, and also inhibits the growth of neuroendocrine tumours through its specific receptors (SSTRs). Somatostatin receptors are G-protein-coupled receptors, which are encoded by five separate genes (SSTR1-5). Short peptide analogues demonstrate specific binding only for the subgroup consisting of SSTR2a, SSTR3 and SSTR5. Moreover, previous studies reported that expression of mRNA for SSTR2a correlated with therapeutic outcome in patients with carcinoid tumours treated with somatostatin analogs.
Purpose
To develop and apply a Real Time Quantitative PCR technique (RT-qPCR) to compare and contrast the mRNA levels of SSTR2a, SSTR3 and SSTR5 in Neuroendocrine Lung Cancer affected patients.
Methods
Peripheral blood samples from 21 neuroendocrine lung cancer affected patients (14 SCLC, 6 LC and 1 LCNEC) subjected to scintigraphy with 111In-DTPA-D-Phe1-octreotide (OctreoScan) and 24 healthy blood donors were investigated by RT-qPCR. mRNA levels for SSTR2a, SSTR3 and SSTR5 were measured in peripheral blood samples with a relative quantification method using plasmid dilutions as calibration curves and GAPDH as reference gene.
Results
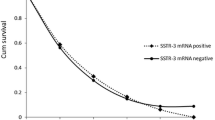
A statistically significant increase in target genes/GAPDH copy number ratio was found for SSTR2a (median 38; IQR 22–141) and SSTR5 (median 51; IQR 19–499) in neuroendocrine lung cancer affected patients as compared with samples from healthy blood donors (P ≤ 0.0003 and P ≤ 0.0005). Since low levels of expression were detected in the control group for all three genes, optimal cut-off values were assessed using ROC curve analyses and were equal to 9.05 for SSTR2a and 16.97 for SSTR5. These cut off values resulted in a sensitivity of 86% (95%IC 65–95) for both markers and a specificity of 83% (95%IC 64–93%) and 79% (95%IC 60–91%) for SSTR2a and SSTR5 respectively. Comparison between OctreoScan results and RT-qPCR analysis demonstrated agreement in 76% of the cases.
Conclusions
Our results suggest that SSTR2a and SSTR5 mRNAs are detectable in peripheral blood of neuroendocrine lung cancer affected patients using real-time quantitative PCR, with a good agreement with OctreoScan. The high sensitivity of this non-invasive molecular technique suggests that this method could represent a useful tool in the clinical management of neuroendocrine lung cancers.

Similar content being viewed by others
References
A.V. Schally, Oncological applications of somatostatin analogues. Cancer Res. 48, 6977–6985 (1988)
C. Capella, P.U. Heitz, H. Höfler, E. Solcia, G. Klöppel, Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 6, 547–560 (1995)
V.E. Gould, W. Jao, G. Chejfec, B.F. Banner, P. Bonomi, Neuroendocrine carcinomas of the gastrointestinal tract. Semin. Diagn. Pathol. 1, 13–18 (1984)
K. Oberg, B. Eriksson, E.T. Janson, The clinical use of interferons in the management of neuroendocrine gastroenteropancreatic tumors. Ann. NY Acad. Sci. 15, 471–478 (1994)
N. Kimura, W. Miura, T. Noshiro, Y. Miura, T. Ookuma, H. Nagura, Ki67 is an indicator of progression of neuroendocrine tumors. Endocr. Pathol. 5, 223–228 (1994)
C. Bruns, G. Weckbecker, F. Raulf, K. Kaupmann, P. Schoeffter, D. Hoyer, H. Lübbert, Molecular pharmacology of somatostatin-receptor subtypes. Ann. NY Acad. Sci. 15, 138–146 (1994)
Y.C. Patel, C.B. Srikant, Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1–5). Endocrinology 135, 2814–2817 (1994)
V. D’Alessandro, L.A. Muscarella, M. Copetti, L. Zelante, M. Carella, G. Vendemiale, Molecular detection of neuron-specific ELAV-like-positive cells in the peripheral blood of patients with small-cell lung cancer. Cell. Oncol. 30, 291–297 (2008)
K. Swerts, B. De Moerloose, C. Dhooge, J. Vandesompele, C. Hoyoux, K. Beiske, Y. Benoit, G. Laureys, J. Philippe, Potential application of ELAVL4 real-time quantitative reverse transcription-PCR for detection of disseminated neuroblastoma cells. Clin. Chem. 52, 438–445 (2006)
P. Goldstraw (ed.), IASLC staging manual in thoracic oncology (Editorial Rx Press, Orange Park, 2009)
M. Pless, C. Waldherr, H. Maecke, C. Buitrago, R. Herrmann, J. Mueller-Brand, Targeted radiotherapy for small cell lung cancer using 90Yttrium-DOTATOC, an Yttrium-labelled somatostatin analogue: a pilot trial. Lung Cancer 45, 365–371 (2004)
G.F. Taboada, R.M. Luque, W. Bastos, R.F. Guimarães, J.B. Marcondes, L.M. Chimelli, R. Fontes, P.J. Mata, P.N. Filho, D.P. Carvalho, R.D. Kineman, M.R. Gadelha, Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur. J. Endocrinol. 156, 65–74 (2007)
M. de Jong, W.A. Breeman, D.J. Kwekkeboom, R. Valkema, E.P. Krenning, Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 42(7), 873–880 (2009)
N. Kimura, M. Pilichowska, F. Date, I. Kimura, M. Schindler, Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin. Cancer Res. 5, 3483–3487 (1999)
H. Pisarek, T. Stepień, R. Kubiak, E. Borkowska, M. Pawlikowski, Expression of somatostatin receptor subtypes in human thyroid tumors: the immunohistochemical and molecular biology (RT-PCR) investigation. Thyroid Res. 27, 1 (2009)
L. Righi, M. Volante, V. Tavaglione, A. Billè, L. Daniele, T. Angusti, F. Inzani, G. Pelosi, G. Rindi, M. Papotti, Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann. Oncol. 21, 548–555 (2010)
G.F. Taboada, R.M. Luque, L.V. Neto, O. Machado Ede, B.C. Sbaffi, R.C. Domingues, J.B. Marcondes, L.M. Chimelli, R. Fontes, P. Niemeyer, D.P. de Carvalho, R.D. Kineman, M.R. Gadelha, Quantitative analysis of somatostatin receptor subtypes (1–5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur J Endocrinol 158, 295–303 (2008)
M. Papotti, S. Croce, M. Bellò, M. Bongiovanni, E. Allìa, M. Schindler, G. Bussolati, Expression of somatostatin receptor types 2, 3 and 5 in biopsies and surgical specimens of human lung tumours. Correlation with preoperative octreotide scintigraphy. Virchows Arch. 439, 787–797 (2001)
A. Schoenfeld, K.H. Kruger, J. Gomm, H.D. Sinnett, J.-C. Gazet, N. Sacks, H.G. Bender, Y. Luqmani, R.C. Coombes, The detection of micrometastases in the peripheral blood and bone marrow of patients with breast cancer using immunohistochemistry and reverse transcriptase polymerase chain reaction for keratin 19. Eur. J. Cancer 33, 854–861 (1997)
R. Schuster, N. Max, B. Mann, K. Heufelder, F. Thilo, J. Gröne, F. Rokos, H.-J. Buhr, E. Thiel, U. Keilholz, Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int. J. Cancer 108(2), 219–227 (2004)
A. Stathopoulou, I. Vlachonikolis, D. Mavroudis, M. Perraki, Ch Kouroussis, S. Apostolaki, N. Malamos, S. Kakolyris, A. Kotsakis, N. Xenidis, D. Reppa, V. Georgoulias, Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J. Clin. Oncol. 20(16), 3404–3412 (2002)
Acknowledgements
The authors wish to thank the patients and the volunteers for their indispensable co-operation. This study was supported by the Italian Ministry of Health (Ricerca Corrente 2010 RC1003MG58). The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lucia Anna Muscarella, Vito D’Alessandro, Vito Michele Fazio, Gianluigi Vendemiale have equally contributed to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
SSTR marker expression also for the different subcategories of lung cancer patients. (DOC 69.5 kb)
Supplemental Figure 1
Standard curves for SSTR2a, SSTR3 and SSTR5 plasmid dilutions. The graph shows the mean of Ct values versus log of plasmid copy numbers, measured in triplicate. Slope and intercept are represented in the equations of the regression lines, together with regression coefficient. (DOC 82 kb)
Rights and permissions
About this article
Cite this article
Muscarella, L.A., D’Alessandro, V., la Torre, A. et al. Gene expression of somatostatin receptor subtypes SSTR2a, SSTR3 and SSTR5 in peripheral blood of neuroendocrine lung cancer affected patients. Cell Oncol. 34, 435–441 (2011). https://doi.org/10.1007/s13402-011-0025-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-011-0025-9