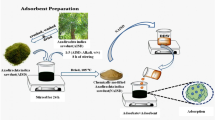
Abstract
Adsorption using sawdust from Malaysian teak wood as adsorbent has been studied in order to remove reactive blue dye from aqueous solutions (AS). The dye removal study comprised of investigation of parameters such as concentration of dye, pH, agitation time, and temperature. Optimization was performed using response surface methodology. Kinetics of adsorption of reactive blue dye with activated sawdust was analyzed using Lagergren’s kinetic models, and it was found that the dye reduction efficiency by activated sawdust followed pseudo-second-order kinetic model. Langmuir and Freundlich’s isotherm models were used for the fitment of batch adsorption experimental data, and it was observed that Langmuir model was found to agree with the values obtained by experimentation based on regression analysis and RMSD values. The study showed that activated sawdust as a promising adsorbent for the reduction of reactive blue dye from AS.
Similar content being viewed by others
Abbreviations
- Q o :
-
Adsorption capacity (mg/g)
- k f :
-
Adsorption capacity (mg/g)
- t :
-
Agitation time (min)
- q :
-
Amount of adsorbate adsorbed (mg/g)
- q e :
-
Amount adsorbed at equilibrium (mg/g)
- b :
-
Constant (L/mg)
- n :
-
Constant (–)
- R L :
-
Dimensionless constant (–)
- c e :
-
Equilibrium concentration of adsorbate (mg/L)
- K 1 :
-
Lagergren’s pseudo-first-order rate constant (min−1)
- K 2 :
-
Lagergren’s pseudo-second-order rate constant (g mg−1 min−1)
References
Bhatnagar A., Jain J.K.: A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Colloid Interface Sci. 281, 49–55 (2005)
Somasekhara Reddy, M.C.; Nirmala, V.; Ashwini, C.: Bengal Gram Seed Husk as an adsorbent for the removal of dyes from aqueous solutions—Batch studies. Arab. J. Chem. (2014). doi:10.1016/j.arabjc.2013.09.029
Clarke, E.A.; Anliker, R.: Organic dyes and pigments. In: Hutzinger, O. (ed.) Anthropogenic compounds. The handbook of environmental chemistry, vol. 3/3A, pp. 181–215. Springer, Berlin, Heidelberg (1980)
Mishra G., Tripathy M.A.: Critical review of the treatments for decolourization of textile effluent. Colourage 40(10), 35–38 (1993)
Deshannavar, U.B.; Ratnamala, G.M.; Kalburgi, P.B.; El-Harbawi, M.; Agarwal, A.; Shet, M.; Teli, M.; Bhandare, P.: Optimization, kinetic and equilibrium studies of disperse yellow 22 dye removal from aqueous solutions using Malaysian teak wood sawdust as adsorbent. Indian Chem. Eng. (2014). doi:10.1080/00194506.2014.987831
Chakraborty S., De S., Das Gupta S., Basu J.K.: Adsorption study for the removal of a basic dye: experimental and modeling. Chemosphere 58, 1079–1086 (2005)
Bhatti H.N., Akhtar N., Saleem N.: Adsorptive removal of methylene blue by low-cost citrus sinensis bagasse: equilibrium, kinetic and thermodynamic characterization. Arab. J. Sci. Eng. 37, 9–18 (2012)
Lim, L.B.L.; Priyantha, N.; Chan, C.M.; Matassan, D.; Chieng, H.I.; Kooh, M.R.R.: Adsorption behavior of methyl violet 2B using duckweed: equilibrium and kinetics studies. Arab. J. Sci. Eng. (2014). doi:10.1007/s13369-014-1224-2
Khan, A.; Rashid, A.; Younas, R.: Adsorption of reactive black-5 by pine needles biochar produced via catalytic and non-catalytic pyrolysis. Arab. J. Sci. Eng. (2015). doi:10.1007/s13369-015-1601-5
Yagub M.T., Sen T.K., Afroze S., Ang H.M.: Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 209, 172–184 (2014)
Langmuir I.: The adsorption of gases on plane surfaces of glass, mica, and platinum. J. Am. Chem. Soc. 40, 1361–1368 (1918)
Freundlich H.: Adsorption in solution. Phys. Chem. Soc. 40, 1361–1368 (1906)
Lagergren S.: Zur theorie der sogenannten adsorption geloster stoffe [About the theory of so called adsorption of soluble substances]. Kungliga Svenska Vetenskapsakademiens Handlingar [K. Sven. Vetenskapsakad. Handl]. 24, 1–39 (1898)
Ho Y.S., Mckay G.: Pseudo-second order model for sorption processes. Process Biochem. 34, 451–465 (1999)
APHA: Standard Methods for the Examination of Water and Wastewater, 20th edn. American Public Health Association, Washington (1980)
Kosmulski M.: The pH-dependent surface charging and the points of zero charge. J. Colloid Interface Sci. 253, 77–87 (2002)
Ofomaja A.E., Ho Y.S.: Effect of temperatures and pH on methyl violet biosorption by Mansonia wood sawdust. Bioresour. Technol. 99, 5411–5417 (2008)
Liu W., Yao C., Wang M., Ji J., Ying L., Fu C.: Kinetics and thermodynamics characteristics of cationic yellow X-GL adsorption on attapulgite/rice hull-based activated carbon nanocomposites. Environ. Prog. Sustain. Energy 32(3), 655–662 (2012)
Ratnamala G.M., Vidya Shetty K., Srinikethan G.: Removal of remazol brilliant blue dye from dye contaminated water by adsorption using red mud: equilibrium, kinetic and thermodynamic studies. Water Air Soil Pollut. 9, 6187–6199 (2012)
Mehdi S.S., Seyed J.J., Omid G., Imsoon K., Seung M.L., Jae K.Y.: Removal of acid blue 113 and reactive black 5 dye from aqueous solutions by activated red mud. J. Ind. Eng. Chem. 20(4), 1432–1437 (2014)
Nevine K.A.: Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 223(1-3), 152–161 (2008)
Mylsamy S., Theivarasu C.: Adsorption of reactive dye using low cost adsorbent: cocoa (Theobroma cacao) shell. World J. Appl. Chem. Environ. Chem. 1(1), 22–29 (2012)
Namasivayam C., Prabha D., Kumutha M.: Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour. Technol. 64(1), 77–79 (1998)
Mckay G.: Use of Adsorbents for the Removal of Pollutants from Wastewater. CRC Press, New York (1996)
Al-Degs Y.S., El-Barghouthi M.I., El-Sheikh A.H., Walker G.M.: Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm. 77, 16–23 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratnamala, G.M., Deshannavar, U.B., Munyal, S. et al. Adsorption of Reactive Blue Dye from Aqueous Solutions Using Sawdust as Adsorbent: Optimization, Kinetic, and Equilibrium Studies. Arab J Sci Eng 41, 333–344 (2016). https://doi.org/10.1007/s13369-015-1666-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-015-1666-1