Abstract
Understanding how invasive predators impact native species is essential for the development of effective control strategies, especially in insular environments where alternative non-native prey species exist. We examined seasonal and spatial shifts in diet of feral cat Felis silvestris catus focusing on the predation on native streaked shearwaters, Calonectris leucomelas, and introduced rats, Rattus rattus and R. norvegicus, which are alternative prey to shearwaters, on Mikura Island, Japan. Streaked shearwaters breed at low elevations on the island from spring to autumn, whereas rats inhabit the island throughout the year, which makes them an alternative prey when native shearwaters are absent. Fecal analysis revealed that feral cats dramatically shifted their diets from introduced rats in winter to streaked shearwaters in seabird-season in low elevation areas of the island, while cats preyed on rats throughout the year at high altitudes on the island. This finding suggests that feral cats selectively prey on shearwaters. This is probably because of their large body size and less cautious behavior, and because introduced rats sustain the cat population when shearwaters are absent. The number of streaked shearwaters killed was estimated to be 313 individuals per cat per year, which represents an indication of top-down effects of feral cats on streaked shearwaters. Further studies on the demographic parameters and interspecific interactions of the three species are required to enable effective cat management for the conservation of streaked shearwaters on this island.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive mammals have been responsible for population declines, local extinctions, and even global extinctions of island species (Diamond 1989; Courchamp et al. 2003). In particular, oceanic island ecosystems, which are often devoid of indigenous mammalian predators, are vulnerable to invasive species because the communities consist of organisms that have no evolutionary defenses against predation (Kier et al. 2009; Medina et al. 2011).
Domestic cats, Felis silvestris catus, have been introduced to islands around the world (Courchamp et al. 2003), and they are one of the most widely established species on islands because they are highly fecund and adaptable to novel environments (Courchamp et al. 2003; Fitzgerald and Turner 2000). Seabirds are occasionally the primary food source of cats where seabird colonies exist (Bonnaud et al. 2011). Some seabirds have evolved in environments free of mammalian predators and thus lack behavioral, morphological, and life-history defenses against mammalian predators, making them one of the taxa most impacted by feral cats (Stattersfield and Capper 2000). Petrels and shearwaters, in particular, are more vulnerable to predation by feral cats because they exhibit low vigilance and agility (Keitt et al. 2002). Seabird populations are also susceptible to invasive predators due to their low fecundity and slow sexual maturation (Moors and Atkinson 1984). In particular, the predation of adult birds can lead to significant population declines or extinctions (Russell 1999).
It should be noted that island ecosystems occasionally harbor introduced species other than feral cats, such as rats. In these situations, the interactions between cats and other introduced species should also be considered, because the elimination of top predators may cause unintended consequences, such as increases in the populations of mesopredators, which is called mesopredator release (Courchamp et al. 1999). Hyperpredation is another type of interaction that occurs between native and multiple introduced species, in which alien rodents increase the density of top predators, maintaining long-lasting impacts of top predators on native species (Courchamp et al. 2000). Therefore, understanding the diets of feral cats is essential for the development of effective control strategies of feral cats in complex food webs that include native and non-native species (Zavaleta et al. 2001).
Our study focused on feral cats on Mikura Island in Japan. Mikura Island is surrounded by sea cliffs up to 500 m in height and is an oceanic island on which no mammalian predators historically occurred. This island harbors the largest breeding population of streaked shearwaters, Calonectris leucomelas, in the world (Oka 2004). Recently, the breeding number of streaked shearwaters has drastically decreased from 1,750,000–3,500,000 in 1978 (Tokyo Metropolitan Government 1980) to 110,000 in 2016 (Biodiversity Center of Japan 2017). The primary reason for this rapid decrease on the island is considered to be predation by feral cats, which are thought to have increased in numbers in recent years (Oka 2019). Predation on streaked shearwaters by cats was recorded by a camera trap (Tokuyoshi et al. 2020), and many carcasses of shearwaters, presumably killed by feral cats, have been found on the island during the shearwater breeding season (Biodiversity Center of Japan 2017; Oka 2019). In addition to feral cats, there are two other non-native mammals on Mikura Island, the black rat, Rattus rattus, and Norway rat, R. norvegicus (Kobayashi and Kikuta 2008; Biodiversity Center of Japan 2017; Azumi et al. 2019). Therefore, it is essential to understand the interspecific relationships between feral cats, non-native rats, and shearwaters on the island.
The diet of feral cats on Mikura Island is expected to change both seasonally and spatially: the shearwaters only inhabit the island from March to November, while they are absent the rest of the year (Oka 2004), and year-round, they are absent from areas with elevations > 600 m (Biodiversity Center of Japan 2013). In winter, when the shearwaters are absent, the feral cats are expected to prey mainly on rats, but in seabird-season, they can choose to feed on streaked shearwaters or rats. If feral cats prefer shearwaters to rats, changing to feed on shearwaters is assumed to have a significant effect on the feeding habits of feral cats.
This study aims to determine how the diets of feral cats fluctuate seasonally and spatially according to the availability of streaked shearwaters. It also discusses the mechanism by which feral cats impact on the streaked shearwater on Mikura Island. Finally, the number of the shearwaters killed by a cat was calculated based on the results of fecal analysis and energetic requirement of cats. The findings of this study are important for informing the management of feral cats on Mikura Island.
Methods
Survey site
Mikura Island (33° 52′ N, 139° 36′ E), which is one of the Izu Islands of Japan, is located about 200 km south-southwest from Tokyo. It is located in a humid temperate climate, the mean monthly temperature is 26.2 °C in August and 9.6 °C in February, and the annual precipitation is about 3000 mm on Miyake Island, which is 20 km north of Mikura Island. Mikura Island has a steep topography, with an area of 20.54 km2, and the highest altitude is 851 m. About 300 villagers inhabit one settlement in the north-northwest of the island. Most of the island from the low to mid-altitude zone is covered with mature ever-green broad-leaved forest, composed mainly of Castanopsis sieboldii, while scrub forests and bamboo bushes dominate in the windswept zone around the top of the island (Kamijo et al. 2001).
The streaked shearwater is a kind of procellariiformes that burrows a nest in the slopes of the forest floor to breed in colonies on islands. Distribution of breeding sites of this species is globally restricted to East Asia (Oka et al. 2002), listed as NT in IUCN Red List. However, this species is locally common and can form large colonies at some breeding sites, which might lead to less awareness of conservation of its local populations (Oka 2019). The shearwater has a long lifespan and slow-reproduction. They start breeding as late as at 6–8 years of age and lay a single egg a year (Oka and Yamamoto 2016). On Mikura Island, their nesting density varies spatially, being the highest in the areas below 400 m in altitude and moderate, with a 75% decrease, at higher altitudes. They do not nest in areas above 600 m in altitude (Biodiversity Center of Japan 2008, 2013, 2017). Hereafter, the period from 1 March to 17 November (262 days), during which streaked shearwaters are present on the island, is called “seabird-season”, and the period from 18 November to 28 February (103 days), during which the shearwater is absent, is called “non-seabird-season” (Oka 2004).
The three alien species, feral cats, black rats, and Norway rats, have become established on Mikura Island with human settlement. Although the two rat species are found at all altitudes, their frequency of appearance is relatively high at low altitudes year-round (Azumi et al. 2019). Other ground-dwelling vertebrates include the native Okada’s blue-tailed skink, Plestiodon latiscutatus, and the Japanese striped snake, Elaphe quadrivirgata, but they are rarely observed in winter. No native mammals or amphibians are distributed on this island (Mikura-shima Village 2007).
Fecal sampling and analysis
Fecal analysis was conducted to determine the seasonal and spatial diets of feral cats. In seabird-season, when shearwaters were present on the island, feces were collected from April to June 2017 from various places except for the residential area, and in winter, when shearwaters were absent, the feces were collected from December 2016 to February 2017. Most of the feces were collected opportunistically during walking surveys on the roads or nature trails in a conservation area that was designated by the local government at Mikura-shima village. Old feces that were broken and lost their shape were excluded from the analysis, but some of the feces may have been left over from the previous season in cases when their shape was well preserved due to low temperature in the winter (Bonnaud et al. 2009). The sampling points were recorded using a GPS device. Some feces were collected from cats trapped in the management project at Mikura-shima village to control feral cats. In such cases, the location where the feral cats were caught was recorded.
All feces were frozen until analysis was conducted in the laboratory. Feces were placed in a 1-mm mesh net and washed in water. Undigested materials such as hairs, feathers, teeth, bones, nails, hard fragments of insects, soft tissues, and debris were separated and were identified, with as much detail as possible, using a stereomicroscope. The prey species found in feral cat feces were classified into six categories: streaked shearwaters, rats, terrestrial birds, insects, other invertebrates, and plants. Hairs and bones of black and Norway rats could not be distinguished by their appearance, so the two rat species were combined and classified as rats.
The minimum number of consumed prey items was determined in each scat if multiple distinctive parts of the same species were found in a scat. If the prey fragments were not countable, all of these were considered to come from a single prey animal. For each identified prey species, the number of individual prey (NI) and frequency of occurrence (%FO), which was the percentage of fecal samples containing a particular prey item, were calculated separately for each season.
To examine whether the altitudinally heterogeneous distribution of the shearwaters affected the diet of feral cats or not, logistic regression analysis was performed for the occurrence of streaked shearwaters and rats in cat feces, using the altitude of the sampling points as an explanatory variable. This analysis was conducted separately for seabird-season and non-seabird-season.
To determine the importance of different prey species, biomass consumed per scat (BM) and the percentage contribution of each prey species (i) to BM (%BMi) were calculated. The BM was calculated using an equation (Bonnaud et al. 2007):
For the streaked shearwaters, instead of using its mean body mass (513 g) (Matsumoto et al. 2012), we assumed that a single meal consumed by a cat is 50% of the body mass of streaked shearwaters (256.5 g) according to Keitt et al. (2002) (Table 1), because bones, feathers, and other parts are left uneaten. For rats and terrestrial birds, it is assumed that their whole body was eaten, according to Bonnaud et al. (2007). The body mass of rats was assumed to be 233 g, which is the average of the two species: the black rat weighs 177 g on average, and the Norway rat weighs 289 g on average (Azumi et al. 2019). Regarding the terrestrial birds, all prey items were assumed to be thrushes, 81 g (Kuroda 1964; Tokuyoshi et al. 2020). Since the invertebrates found in the feces samples were negligible in their energy contents, being much smaller in size and lower in frequency than those of vertebrate preys, BM and %BMi were calculated for streaked shearwaters, rats, and terrestrial birds.
To calculate the number of each prey animal species killed per unit time, we used the estimated daily consumption (DCB) and body mass of each prey item. The DCB can be estimated by an allometric equation (Nagy 1987; Keitt et al. 2002):
where, the coefficient 2.86 is included to account for the 65% water content of prey, and the coefficient 18 represents the mean energy content (kJ) of metabolizable energy of dry prey per gram (Nagy 1987; Keitt et al. 2002). The average body mass of 14 feral cats captured on Mikura Island in February 2017 was 3.4 kg (2.0–4.4 kg). Using the average body mass of cats, DCB of feral cats on Mikura Island was estimated to be 395 g. The number of each prey item (i) killed per cat in each season (Ni,season) was calculated by the following equation (Keitt et al. 2002):
where Daysseason for seabird-season and non-seabird-season were 262 and 103 days, respectively. For Body_massi, 256.5 g was used for the streaked shearwaters while mean body mass was used for rats and terrestrial birds. Finally, the number of each prey item killed yearly per cat (Ni,year) was calculated as the sum of the estimates in both seasons.
Results
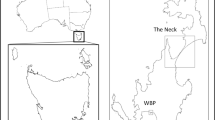
In total, 168 fecal samples (82 in seabird-season and 86 in non-seabird-season) (Fig. 1) were collected. Among these samples, altitude information was available for 165 (82 in seabird-season and 83 in non-seabird-season). The most frequent prey found in seabird-season was streaked shearwaters (78.3%), followed by rats (27.7%) (Table 1). Among the plant materials (14.5%), the cherry, Prunus speciosa, (9.6%) showed a relatively higher frequency. The frequencies of insects (2.4%) and other invertebrates (1.2%) were quite low. However, rats (91.9%) became the most frequent prey species in non-seabird-season. Terrestrial birds (20.9%) were the second most frequent, and one of them was probably a thrush, judging from the size of their legs. Plants (10.5%), streaked shearwaters (5.8%), and other invertebrates (1.2%) followed (Fig. 2). The frequency of rats was significantly higher in non-seabird-season (X-squared = 69.98, p < 0.01), while that of streaked shearwaters was significantly higher in seabird-season (X-squared = 88.53, p < 0.01).
The occurrence of rats in fecal samples increased significantly with increasing altitude in seabird-season (z value = 3.56, p < 0.01) (Fig. 3a). However, the occurrence of streaked shearwaters decreased with increasing altitude (z value = − 3.76, p < 0.01) (Fig. 3b). In non-seabird-season, neither rats (p = 0.73) nor streaked shearwaters (p = 0.83) showed any relationship with altitude (Fig. 3c, d).
The result of logistic regression analysis of (a) rats and (b) streaked shearwaters in seabird-season, and (c) rats and (d) streaked shearwaters in non-seabird-season, using the occurrence in each fecal sample as the objective variable and the altitude of the sampling point as the explanatory variable
In seabird-season, %BM was most heavily weighted by streaked shearwaters (75.7%), followed by rats (24.3%). In non-seabird-season, rats were the highest %BM (87.3%), followed by streaked shearwaters (5.9%), and terrestrial birds (6.7%) (Fig. 4). Thus, both %FO and %BM showed a similar seasonal tendency, except that %BM of terrestrial birds was much lower than %FO. Throughout the year, the number of rats found in one scat was mostly one individual except for two fecal samples in non-seabird-season with two rat individuals.
Numbers of prey species killed yearly per cat was estimated to be 313 individuals in the streaked shearwaters, 230 in rats, and 27 in terrestrial birds (Table 1).
Discussion
Our study demonstrated a clear seasonal dietary shift in feral cats on Mikura Island from shearwaters in seabird-season to rats in non-seabird-season, both in the frequency of occurrence and contribution of biomass. Adult streaked shearwaters are a worthwhile target to prey in terms of their size and behavior, as they mostly sit on the ground, move slowly on foot, and occur at high population densities because they breed in colonies. Predation on chicks in burrows may also be easy, as cats can enter burrows to take them (Shiozaki et al. 2014). Therefore, streaked shearwaters are considered to be a good food resource for feral cats. However, cats were found to depend on rats in non-seabird-season (Figs. 2 and 4), and rats are resident throughout a year. The reason why some shearwaters were found in non-seabird-season feces is that the decomposition rate of cat feces was slowed by lower temperatures so that some feces defecated in seabird-season were collected during non-seabird-season. Therefore, we conclude that feral cats on Mikura Island are a facultative specialist (Malo et al. 2004; Lozano et al. 2006) that mostly preyed on shearwaters, and some rats, in seabird-season, while they depended mostly on rats, and some terrestrial birds, in winter.
We have also shown that feral cats preyed more on rats at higher altitudes, while they preyed more on streaked shearwaters at lower altitudes (Fig. 3). This implies that rats were the primary prey item for feral cats even in seabird-season, where the local density of streaked shearwaters was low or zero. However, there was no such relationship between fecal contents and altitude in winter when streaked shearwaters were absent. These results indicate that feral cats change their food habits, not only with season but also with altitude. It appears that the seasonal and spatial food habits of feral cats were driven by the presence or absence of streaked shearwaters, and the feral cats selectively preyed on shearwaters and complementarily preyed on rats when shearwaters were absent. As Mikura Island has areas without shearwaters, even in seabird-season, this allowed us to separate the effect of the presence or absence of streaked shearwaters and other unknown seasonal effects, despite the study site being a single island system.
Bonnaud et al. (2011) reviewed the diets of cats on islands and identified the four issues that should be addressed: (1) evaluating diets during the presence of “temporary” prey, such as seabirds, (2) collecting a sufficient number of samples (> 100), (3) evaluating the primary prey categories from both frequency of prey occurrence and prey biomass or relative abundance, and (4) obtaining information in previously understudied insular regions, including Japan. Our study satisfies all of the above requirements and clarified that feral cats on Mikura Island prefer shearwaters as prey when they are available, but they are obliged to shift their diet to invasive rats when shearwaters are unavailable. Therefore, feral cats are considered to exert strong predation pressure on streaked shearwaters due to the presence of alternative prey. Such processes are also documented on other islands, indicating that even small populations of seabirds can be led to complete extinction through hyperpredation (Keitt et al. 2002; Courchamp et al. 1999).
We revealed that a cat was estimated to consume 313 streaked shearwaters per year on Mikura Island, followed by 230 rats and 27 terrestrial birds (Table 1). Keitt et al. (2002) showed two independent estimates of monthly cat predation on 40.5 and 45.0 black-vented shearwaters, Puffinus opisthomelas, on Natividad Island. Our estimate, which corresponds to monthly (for 30 days) 35.0 shearwaters in seabird-season or monthly 26.1 individuals on a yearly average, was smaller to this previous study. Considering that cat consumption of rats is complementarily higher on Mikura Island than on Natividad Island, it may be a realistic estimate for assessing cat consumption. On the other hand, the number of prey individuals killed by cat does not necessarily reflect the impact of cats on prey population (Bonnaud et al. 2011). Even if the number of shearwaters killed by cats is small, the top-down effects on shearwater populations may be strong. For example, yearly per cat predation on Townsend’s shearwaters, Puffinus auricularis auricularis, was 71.5 individuals on Socorro Island (Martínez-Gómez and Jacobsen 2004), and that on Yelkouan shearwaters, Puffinus yelkouan, was 21.5 on Port-Cros Island (Bonnaud et al. 2009), and both shearwater populations were predicted to become extinct by cat predation using numerical simulations. In addition, Oka and Yamamoto (2016) reported that carcasses of the streaked shearwaters with external wound, which might have been killed by feral cats, were frequently found on forest floor on Mikura Island. This indicates that more shearwaters are killed than energy requirement of cats through predation without consumption, i.e., surplus killing (Peck et al. 2008; Towns et al. 2011). Therefore, it is necessary to consider the possibility of underestimation of top-down effects induced by cats in the future.
When implementing cat population control, we have to consider the possibility of mesopredator release (Courchamp et al. 1999; Ringler et al. 2015) that causes unexpected negative impact on the shearwaters through increased number of rats. However, direct predation of rats on the shearwaters or their eggs have rarely been observed on Mikura Island, even under intensive fieldworks (Tokuyoshi, personal communications). Moreover, since we observed rats feeding on carcasses of streaked shearwaters killed by feral cats using a video camera trap (Azumi et al. unpublished), cats may indirectly supply food to rats through providing uneaten carcasses of streaked shearwaters. Quantifying whether such an indirect effect could contribute to the mesopredator release may provide new insights into the context-dependency in this phenomenon (Rayner et al. 2007; Ritchie and Johnson 2009; Bonnaud et al. 2010; Watari et al. 2011; Ringler et al. 2015). However, the current density of shearwaters is already so low that a slight increase in rats could have an irreversible impact, given the potentially low population growth rate of this pelagic bird (Oka and Yamamoto 2016). Further studies on the demographic parameters and interspecific interactions of the four species are required to make recommendations for the effective management of invasive species on Mikura Island to enable the conservation of the declining population of threatened streaked shearwaters.
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Azumi S, Oka N, Watari Y (2019) Ecological aspects of the invasive rats, Rattus rattus and R. norvegicus on Mikura Island, Japan. Honyurui Kagaku (Mammalian Science) 59:85–91 (in Japanese with English summary)
Biodiversity Center of Japan (2008) Monitoring site 1000 seabird investigation report fiscal year 2007. Nature Conservation Bureau, Ministry of the Environment, Fujiyoshida, Japan (in Japanese)
Biodiversity Center of Japan (2013) Monitoring site 1000 seabird investigation report fiscal year 2012. Nature Conservation Bureau, Ministry of the Environment, Fujiyoshida, Japan (in Japanese)
Biodiversity Center of Japan (2017) Monitoring site 1000 seabird investigation report fiscal year 2016. Nature Conservation Bureau, Ministry of the Environment, Fujiyoshida, Japan (in Japanese)
Bonnaud E, Bourgeois K, Vidal E, Kayser Y, Tranchant Y, Legrand J (2007) Feeding ecology of a feral cat population on a small Mediterranean island. J Mammal 88:1074–1081
Bonnaud E, Bourgeois K, Vidal E, Legrand J, Le Corre M (2009) How can the Yelkouan shearwater survive feral cat predation? A meta-population structure as a solution? Popul Ecol 51:261–270
Bonnaud E, Zarzoso-Lacoste D, Bourgeois K, Ruffino L, Legrand J, Vidal E (2010) Top-predator control on islands boosts endemic prey but not mesopredator. Anim Conserv 13:556–567
Bonnaud E, Medina FM, Vidal E, Nogales M, Tershy B, Zavaleta E, Donlan CJ, Keitt B, Le Corre M, Horwath SV (2011) The diet of feral cats on islands: a review and a call for more studies. Biol Invasions 13:581–603
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68:282–292
Courchamp F, Langlais M, Sugihara G (2000) Rabbits killing birds: modelling the hyperpredation process. J Anim Ecol 69:154–164
Courchamp F, Chapuis JL, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Diamond J (1989) Overview of recent extinctions. In: Western D, Pearl MC (eds) Conservation for the Twenty-first Century. Oxford University Press, Oxford, pp 37–41
Fitzgerald BM, Turner DC (2000) Hunting behaviour of domestic cats and their impact on prey populations. In: Turner DC, Bateson P (eds) The domestic cat: the biology of its behaviour, 2nd edn. Cambridge University Press, Cambridge, pp 151–175
Kamijo T, Isogai T, Hoshino Y, Hakamada H (2001) Altitudinal zonation and structure of warm-temperate forests on Mikura Island, Izu Islands, Japan. Veg Sci 18:13–22
Keitt BS, Wilcox C, Tershy BR, Croll DA, Donlan CJ (2002) The effect of feral cats in the population viability of black-vented shearwaters (Puffinus opisthomelas) on Natividad Island, Mexico. Anim Conserv 5:217–223
Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, Nowicki C, Mutke J, Barthlott W (2009) A global assessment of endemism and species richness across island and mainland regions. PNAS 106:9322–9327
Kobayashi M, Kikuta A (2008) Distribution and food habits of the roof rat Rattus rattus in the recovering Sasa jotanii population 4 years after the monocarpic mass flowering in Mt. Oyama, Mikurajima Island, Izu Islands, Japan. Bamboo J 25:1–8 (in Japanese)
Kuroda N (1964) Analysis of variation by sex, age, and season of body weight, fat and some body parts in the Dusky Thrush, wintering in Japan: a preliminary study. Misc Rep Yamashina’s Inst Ornith Zool 4:91–105
Lozano J, Moleón M, Virgós E (2006) Biogeographical patterns in the diet of the wildcat, Felis silvestris Schreber, in Eurasia: factors affecting the trophic diversity. J Biogeogr 33:1076–1085
Malo AF, Lozano J, Huertas DL, Virgos E (2004) A change of diet from rodents to rabbits (Oryctolagus cuniculus). Is the wildcat (Felis silvestris) a specialist predator? J Zool 263:401–407
Martínez-Gómez JE, Jacobsen JK (2004) The conservation status of Townsend’s shearwater Puffinus auricularis auricularis. Biol Conserv 116:35–47
Matsumoto K, Oka N, Ochi D, Muto F, Satoh TP, Watanuki Y (2012) Foraging behavior and diet of streaked shearwaters Calonectris leucomelas rearing chicks on Mikura Island. Ornithol Sci 11:9–19
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Donlan CJ, Keitt BS, Corre ML, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Chang Biol 17:3503–3510
Mikura-shima Village (2007) Field guide of plants & animals in Mikura Island. Mikura-shima Village. Mikura-shima, Japan
Moors PJ, Atkinson IAE (1984) Predation on seabirds by introduced animals, and factors affecting its severity. In: Croxall JP, PGH E, Schreiber RW (eds) Vol. 2: Status and conservation of the world’s seabirds. ICPB Technical Publications, Cambridge, pp 667–690
Nagy KA (1987) Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr 57:111–128
Oka N (2004) The distribution of streaked shearwater colonies, with special attention to population size, area of sea where located and surface water temperature. J Yamashina Inst Ornithol 35:164–188 (in Japanese with English summary)
Oka N (2019) Sharp decrease of the world’s largest breeding population of the streaked shearwaters affected by the feral cats on Mikura Island, Tokyo. J Jpn Wildl Res Soc 44:65–72 (in Japanese)
Oka N, Yamamoto M (2016) Feral cat issues on the largest population of streaked shearwaters in Japan. Gekkan kaiyo 48:405–408 (in Japanese)
Oka N, Suginome H, Jida N, Maruyama N (2002) Chick growth and fledging performance of streaked shearwaters Calonectris leucomelas on Mikura Island for two breeding seasons. J Yamashina Inst Ornithol 34:39–59
Peck DR, Faulquier L, Pinet P, Jaquemet S, Le Corre M (2008) Feral cat diet and impact on sooty terns at Juan de Nova Island, Mozambique Channel. Anim Conserv 11:65–74
Rayner MJ, Hauber ME, Imber MJ, Stamp RK, Clout MN (2007) Spatial heterogeneity of mesopredator release within an oceanic island system. Proc Natl Acad Sci 104:20862–20865
Ringler D, Russell JC, Le Corre M (2015) Trophic roles of black rats and seabird impacts on tropical islands: mesopredator release or hyperpredation? Biol Conserv 185:75–84
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Russell RW (1999) Comparative demography and life history tactics of seabirds: implications for conservation and marine monitoring. In: Musick JA (ed) Life in the slow lane: ecology and conservation of long-lived marine animals. American Fisheries Society, Bethesda, pp 51–76
Shiozaki T, Shirai M, Osugi M, Yamamoto M, Yoda K (2014) Predation by feral cat on streaked shearwater chicks on Awashima. Jpn J Ornithol 63:75–78 (in Japanese)
Stattersfield AJ, Capper DR (2000) Threatened birds of the world. Lynx Edicions and BirdLife International, Barcelona
Tokuyoshi M, Oka N, Watari Y (2020) Domestic cat catching the endangered Izu thrush revealed by camera trap on Mikura Island, Japan. Honyurui Kagaku (Mammalian Science) 60:237–241 (in Japanese with English summary)
Tokyo Metropolitan Government (1980) Survey report of streaked shearwater. Bureau of Industrial and Labor Affairs, Tokyo, Japan (in Japanese)
Towns DR, Byrd GV, Jones HP, Rauzon MJ, Russell JC, Wilcox C (2011) Impacts of introduced predators on seabirds. In: Mulder CPH, Anderson WB, Towns DR, Bellingham PJ (eds) Seabird islands: ecology, invasion, and restoration. Oxford University Press, New York, pp 56–90
Watari Y, Caut S, Bonnaud E, Bourgeois K, Courchamp F (2011) Recovery of both a mesopredator and prey in an insular ecosystem after the eradication of rodents: a preliminary study. In: Veitch CR, Clout MN, Towns DR (eds) Island invasives: eradication and management. IUCN, Gland, Switzerland, pp 377–383
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16:454–459
Acknowledgments
We are especially grateful to Mr. Hisao Hirose, the mayor, and officials of Mikura-shima village office and the staff of Mikura Island Tourist Information Center including Mr. Kazunobu Kogi who helped with our field works. We also thank Ms. Mio Yanase, Mr. Yoshio Tamura, veterinarian, and Mr. Yuichi Inamura of Tokyo University of Agriculture for helping us to collect some cat feces samples. We also thank Dr. Kazuto Kawakami of Forestry and Forest Products Research Institute for help in making figures. We got permissions of entering conservation area and taking feces out of the island from Mikura-shima village office.
Funding
This study was partly supported by the Environment Research and Technology Development Fund (JPMEERF20204006) of the Environmental Restoration and Conservation Agency of Japan.
Author information
Authors and Affiliations
Contributions
Y. Watari and N. Oka designed the concept and Y. Watari and T. Miyashita managed the project. S. Azumi and N. Oka conducted collecting samples in the field. S. Azumi conducted analysis and Y. Watari, N. Oka, and T. Miyashita interpreted the results. S. Azumi made manuscript with critical revises by Y. Watari, N. Oka, and T. Miyashita. All authors approved the final version for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was conducted with permission from Mikura-shima village office to enter the conservation area.
Consent to participate
Not applicable
Consent for publication
Not applicable
Code availability
Not applicable
Additional information
Communicated by: Krzysztof Schmidt
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azumi, S., Watari, Y., Oka, N. et al. Seasonal and spatial shifts in feral cat predation on native seabirds vs. non-native rats on Mikura Island, Japan. Mamm Res 66, 75–82 (2021). https://doi.org/10.1007/s13364-020-00544-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-020-00544-5