Abstract
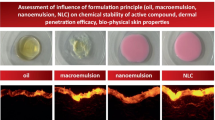
The objective of this study was to explore the oral route as a viable potential for the skin deposition of a novel diindolylmethane derivative (DIM-D) for chemoprevention activity. Various lipid-based oral delivery systems were optimized and compared for enhancing DIM-D’s oral bioavailability and skin deposition. Preformulation studies were performed to evaluate the log P and solubility of DIM-D. Microsomal metabolism, P-glycoprotein efflux, and caco-2 monolayer permeability of DIM-D were determined. Comparative evaluation of the oral absorption and skin deposition of DIM-D-loaded various lipid-based formulations was performed in rats. DIM-D showed pH-dependent solubility and a high log P value. It was not a strong substrate of microsomal degradation and P-glycoprotein. SMEDDs comprised of medium chain triglycerides, monoglycerides, and kolliphor-HS15 (36.70 ± 0.42 nm). SNEDDs comprised of long chain triglycerides, cremophor RH40, labrasol, and TPGS (84.00 ± 14.14 nm). Nanostructured lipid carriers (NLC) consisted of compritol, miglyol, and surfactants (116.50 ± 2.12 nm). The blank formulations all showed >70 % cell viability in caco-2 cells. Differential Scanning Calorimetry confirmed the amorphization of DIM-D within the lipid matrices while Atomic Force Microscopy showed particle size distribution similar to the dynamic light scattering data. DIM-D also showed reduced permeation across caco-2 monolayer that was enhanced (p < 0.05) by SNEDDs in comparison to SMEDDs and NLC. Fabsolute for DIM-D SNEDDs, SMEDDs, and NLC was 0.14, 0.04, and 0.007, respectively. SNEDDs caused 53.90, 11.32, and 15.08-fold more skin deposition of DIM-D than the free drug, SMEDDs, and NLC, respectively, at 2 h following oral administration and shows a viable potential for use in skin cancer chemoprevention.

ᅟ







Similar content being viewed by others
References
Patel AR et al. Pharmacokinetic evaluation and in vitro-in vivo correlation (IVIVC) of novel methylene-substituted 3,3' diindolylmethane (DIM). Eur J Pharm Sci. 2012;46(1–2):8–16.
Vanderlaag K et al. Inhibition of breast cancer cell growth and induction of cell death by 1,1-bis(3'-indolyl)methane (DIM) and 5,5'-dibromoDIM. Cancer Lett. 2006;236(2):198–212.
Rahimi M, Huang KL, Tang CK. 3,3'-Diindolylmethane (DIM) inhibits the growth and invasion of drug-resistant human cancer cells expressing EGFR mutants. Cancer Lett. 2010;295(1):59–68.
Boakye CH et al. Chemoprevention of skin cancer with 1,1-Bis (3'-indolyl)-1-(aromatic) methane analog through induction of the orphan nuclear receptor, NR4A2 (Nurr1). PLoS One. 2013;8(8):e69519.
Boakye CH et al. Enhanced percutaneous delivery of 1, 1-bis (3-indolyl)-1-(p-chlorophenyl) methane for skin cancer chemoprevention. J Biomed Nanotechnol. 2015;11(7):1269–81.
Lipinski CA et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26.
Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–41.
Dufek MB, Bridges AS, Thakker DR. Intestinal first-pass metabolism by cytochrome p450 and not p-glycoprotein is the major barrier to amprenavir absorption. Drug Metab Dispos. 2013;41(9):1695–702.
Dufek MB et al. P-glycoprotein increases portal bioavailability of loperamide in mouse by reducing first-pass intestinal metabolism. Drug Metab Dispos. 2013;41(3):642–50.
Usansky HH, Hu P, Sinko PJ. Differential roles of P-glycoprotein, multidrug resistance-associated protein 2, and CYP3A on saquinavir oral absorption in Sprague-Dawley rats. Drug Metab Dispos. 2008;36(5):863–9.
Fahr A, Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4(4):403–16.
Yang L et al. Enhancement the oral bioavailability of praziquantel by incorporation into solid lipid nanoparticles. Pharmazie. 2009;64(2):86–9.
Li H et al. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–44.
Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev. 2008;60(6):734–46.
O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15(5):405–15.
Gao P et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98.
Cerpnjak K et al. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharma. 2013;63(4):427–45.
Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–55.
Muller RH et al. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59(6):522–30.
Fang CL, Al-Suwayeh SA, Fang JY. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41–55.
Korang-Yeboah M, Gorantla Y, Paulos SA, Sharma P, Chaudhary J, Palaniappan R. Polycaprolactone/maltodextrin nanocarrier for intracellular drug delivery: formulation, uptake mechanism, internalization kinetics, and subcellular localization. Int J Nanomedicine. 2015;10:4763–81.
Sohlenius-Sternbeck AK et al. Evaluation of the human prediction of clearance from hepatocyte and microsome intrinsic clearance for 52 drug compounds. Xenobiotica. 2010;40(9):637–49.
Dixit AR, Rajput SJ, Patel SG. Preparation and bioavailability assessment of SMEDDS containing valsartan. AAPS PharmSciTech. 2010;11(1):314–21.
Patel AR et al. Evaluation of spray BIO-Max DIM-P in dogs for oral bioavailability and in Nu/nu Mice bearing orthotopic/metastatic lung tumor models for anticancer activity. Pharm Res. 2015;32:2292–300.
Patel AR, Chougule M, Singh M. EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer. Pharm Res. 2014;31(10):2796–809.
Boakye CH, Patel K, Singh M. Doxorubicin liposomes as an investigative model to study the skin permeation of nanocarriers. Int J Pharm. 2015;489(1):106–16.
Marepally S et al. Topical administration of dual siRNAs using fusogenic lipid nanoparticles for treating psoriatic-like plaques. Nanomedicine (London). 2014;9(14):2157–74.
Marepally S et al. Design, synthesis of novel lipids as chemical permeation enhancers and development of nanoparticle system for transdermal drug delivery. PLoS One. 2013;8(12):e82581.
Somagoni J et al. Nanomiemgel-A novel drug delivery system for topical application-in vitro and in vivo evaluation. PLoS One. 2014;9(12):e115952.
Fulzele SV et al. Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm Res. 2006;23(9):2094–106.
Amidon GL et al. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Patel K, Sarma V, Vavia P. Design and evaluation of lumefantrine—oleic acid self nanoemulsifying ionic complex for enhanced dissolution. Daru. 2013;21(1):27.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and 'self-microemulsifying' drug delivery systems. Eur J Pharm Sci. 2000;11(Suppl 2):S93–8.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Porter CJ, Wasan KM, Constantinides P. Lipid-based systems for the enhanced delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 2008;60(6):615–6.
Porter CJ et al. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60(6):673–91.
Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16(4–5):237–46.
Seeballuck F, Ashford MB, O'Driscoll CM. The effects of pluronics block copolymers and cremophor EL on intestinal lipoprotein processing and the potential link with P-glycoprotein in Caco-2 cells. Pharm Res. 2003;20(7):1085–92.
Beloqui A et al. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J Control Release. 2013;166(2):115–23.
Patel K et al. Oral delivery of paclitaxel nanocrystal (PNC) with a dual Pgp-CYP3A4 inhibitor: preparation, characterization and antitumor activity. Int J Pharm. 2014;472(1–2):214–23.
Leveque D, Jehl F. P-glycoprotein and pharmacokinetics. Anticancer Res. 1995;15(2):331–6.
Werle M. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm Res. 2008;25(3):500–11.
Di L et al. Optimization of a higher throughput microsomal stability screening assay for profiling drug discovery candidates. J Biomol Screen. 2003;8(4):453–62.
Li X et al. Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech. 2010;11(2):672–8.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Agrawal AG, Kumar A, Gide PS. Self emulsifying drug delivery system for enhanced solubility and dissolution of glipizide. Colloids Surf B: Biointerfaces. 2015;126:553–60.
Porter CJ et al. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm Res. 2004;21(8):1405–12.
Patel K et al. Medium chain triglyceride (MCT) rich, paclitaxel loaded self nanoemulsifying preconcentrate (PSNP): a safe and efficacious alternative to Taxol. J Biomed Nanotechnol. 2013;9(12):1996–2006.
Goddeeris C, Coacci J, Van den Mooter G. Correlation between digestion of the lipid phase of smedds and release of the anti-HIV drug UC 781 and the anti-mycotic drug enilconazole from smedds. Eur J Pharm Biopharm. 2007;66(2):173–81.
Fatouros DG et al. Clinical studies with oral lipid based formulations of poorly soluble compounds. Ther Clin Risk Manag. 2007;3(4):591–604.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76.
Varma MV, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005;25(4–5):445–53.
Fernandez S et al. In vitro gastrointestinal lipolysis of four formulations of piroxicam and cinnarizine with the self emulsifying excipients labrasol and gelucire 44/14. Pharm Res. 2009;26(8):1901–10.
Acknowledgments
The authors would want to express their gratitude to Dr. Jagan M. Somagoni for his contribution to this study.
This project was supported by the NIH Minority Biomedical Research Support (MBRS)-SC1 program [Grant # SC1 GM092779-01; MS] and the National Institute of Minority Health and Health Disparities (NIMHD) NIMHD P20 [Grant # 1P20 MD006738-03; MS].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Boakye, C.H.A., Patel, K., Patel, A.R. et al. Lipid-based oral delivery systems for skin deposition of a potential chemopreventive DIM derivative: characterization and evaluation. Drug Deliv. and Transl. Res. 6, 526–539 (2016). https://doi.org/10.1007/s13346-016-0302-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0302-2