Abstract
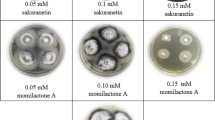
Silicon (Si) has been exploited for its beneficial effects in terms of disease control in many plants. Added Si was found to accumulate beneath the cuticle and act as a barrier against pathogen penetration, and also induce biochemical defense responses in plants. This study investigated the effect of soluble silicon (Si) supply on biochemical defense responses against powdery mildew pathogen (Erysiphe sp.) in bitter gourd (Momordica charantia L.), an intermediate Si accumulator. Supplying the sand-based growth medium with 200 ppm potassium silicate (Si+) significantly reduced the severity of powdery mildew infections, elevated the activities of enzymes peroxidase, polyphenol oxidase and pathogenesis-related proteins; chitinase and β-1,3-glucanase in bitter gourd leaves after challenged by Erysiphe sp. compared with those grown in control mix (Si−). After 7 weeks growth in Si-amended medium, leaves accumulated nearly seven times as much silicon (3.36 % dry weight) as those grown in control mix (0.44 % dry weight). The total Si content in leaves gradually decreased after the Si amendment was ceased. Si + plants exhibited a stronger antifungal activity against Cladosporium cladosporioides. Three zones with antifungal activity were revealed after separation of methanolic leaf extracts on thin-layer chromatography plates, out of which one compound was found to have induced by Si and/or Erysiphe infection, and another induced only by Si amendment. These results suggest that Si would play an active role in strengthening resistance in bitter gourd plants against powdery mildew by stimulating expression of several biochemical defense reactions.







Similar content being viewed by others
References
Adikaram NKB, Ratnayake Bandara BM (1998) Methodology for studying defense mechanisms against fungal pathogens: An overview. In: Johnson GI, Highley E, Joyce DC (eds) Disease resistance in fruit, 18–21 May 1997. Canberra, ACIAR Proceedings No 80 (Xiii), Chaiang Mai, Thailand, pp. 177–185
Adikaram NKB, Karunanayake LC, Abayasekara CL (2010) The role of pre-formed antifungal substances in the resistance of fruits to postharvest pathogens. In: Gullino L, Prusky D (eds) Postharvest Pathology. Springer, New York, pp. p1–13
Al-aghabary K, Zhu Z, Shi Q (2004) Influence of silicon supply on chlorophyll content, chlorophyll florescence and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 27:2101–2115
Anon (1990) Bitter gourd- Crop Recommendations Technoguide. Department of Agriculture Sri Lanka 69–73
Bowen P, Menzies J, Ehret D, Samuels L, Glass ADM (1992) Soluble silicon sprays inhibit powdery mildew development on grape leaves. J. Am Soc Hortic Sci 117:906–912
Bradford MM (1976) A rapid and sensitive method for the estimation of microgram quantities of protein utilizing the principle of protein dye binding. Annal Biochem 72:248–254
Buck GB, Korndorfer G H, Datnoff LE (2010) Extractors for estimating plant available silicon from potential silicon fertilizer sources. J Plant Nutrition 34:2, 272–282
Cai KZ, Gao D, Luo SM, Zeng RS, Yang JY, Zhu XY (2008) Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol Plant 34:324–333
Cai KZ, Gao D, Chen J, Luo S (2009) Probing the mechanism of silicon mediated pathogen resistance. Plant signal behave 4:1–3
Cherif M, Asselin A, Belanger RR (1994) Defence response induced by soluble silicon in cucumber roots infected by Pythium sp. Phytopathol 84(03):236–242
Dallagnol LJ, Rodrigues FA, Da Matta FM, Mielli MVB, Pereira SC (2011) Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice-Bipolaris oryzae interaction. Phytopathol 101:92–104
Dann EK, Deverall BJ (2000) Activation of systemic disease resistance in pea by an avirulent bacterium or a benzothiadiazole, but not by a fungal leaf spot pathogen. Plant Pathol 49:324–332
Dann EK, Muir S (2002) Peas grown in media with elevated plant-available silicon levels have higher activities of chitinase and ß-1–3-glucanase, are less susceptible to a fungal leaf spot pathogen and accumulate more foliar silicon. Australasian Plant Pathol 31:9–13
Datnoff LE, Snyder GH, Deren CW (1992) Influence of silicon fertilizer grades on blast and brown spot development and yields of rice. Plant Dis 76:1182–1184
Datnoff LE, Rodrigues FA, Seebold KW (2009) Silicon and Plant Disease In: Mineral Nutrition and Plant disease. APS Press, USA. 233–246
Dharmakeerthi RS, Indraratne SP, Kumaragamage D (2007) Manual of soil sampling and analysis. Special Publication No.10, Soil Science Society of Sri Lanka. Pp 45–46
Elliot CL, Snyder G (1991) Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J Agric and Food Chem 39:1118–1119
Esteban-Carrasco AM, Lopez-Serrano JM, Zapata B, Sabater Martin M (2001) Oxidation of phenolic compoundsfrom Aloe barbadensis by peroxidase activity:Possible involvement in defence reactions. Plant Physiol Biochem 39:521–527
Fawe A, Abou-Zaid M, Menzies JG, Bélanger RR (1998) Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathol 88:396–401
Fawe A, Menzies JG, Cherif M, Bélanger RR (2001) Silicon and disease resistance in dicotyledons. In: Silicon in Agriculture (eds. Datnoff LE, Snyder GH Korndorfer GH) Elsevier Science B.V. pp 159–169
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxident machinery in abiotic stress tolrance in crop plants. Plant Physiol Biochem 48:909–930
Grubben GJH, Chigumira, NF (2004) Cucurbita moschata Duchesne. In: (eds. Grubben GJH and Denton OA) PROTA 2: Vegetables/Légumes PROTA, Wageningen, The Netherlands pp384–388
Heath MC (1981) Insoluble silicon in necrotic cowpea cells infection with an incompatible isolate of the cowpea rust fungus. Physiol Plant Pathol 19:273–276
Heine G, Tikum G, Horst WJ (2005) Silicon nutrition of tomato and bittergourd with special emphasis on silicon distribution in root fractions. J Plant Nutr Soil Sc. 168:600–666
Heine G, Tikum G, Horst WJ (2007) The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J Exp Bot 58:3 569–577
Jaiti F, Verdeil JL, El-hadrami I (2009) Effect of jasmonic acid on the induction of polyphenol oxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f. sp. albedinis. Physiol Mol Plant Pathol 24:84–90
Jones LHP, Handreck KA (1967) Silica in soils, plants and animals. Adv Agron 19:107–149
Kablan L, Lagauche A, Delvaux B, Legreve A (2012) Silicon reduces black sigatoka development in banana. Plant Dis 96:273–278
Kanto T (2002) Research of silicate for improvement of plant defence against pathogens in Japan. In: Proceedings of the second silicon in agriculture Conference (Matoh T, Ed.) Pres-Net, Kyoto, Japan 22–26
Kim SG, Kim KW, Park EW, Choi D (2002) Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathol 92:1095–1103
Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164:1626–1638
Lewin J, Reimann, Reimann BEF (1969) Silicon and plant growth. Annu Rev Plant Physiol 20:289–304
Liang YC, Sun WC, Si J, Romheld V (2005) Effects of foliar- and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol 54:678–685
Liu J, Chen J, Wang C, Qui M (2009) New cucurbitane triterpenoids and steroidal glycoside from Momordica charantia. Molecules 14:4804–4813
Ma JF. and Takahashi E ( 2002). Soil, Fertilizer and Plant Silicon Research in Japan, Elsevier 1–281
Ma JF, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. In: Silicon in Agriculture (eds. Datnoff LE, Snyder GH Korndorfer GH) Elsevier Science B.V. pp 17–39
Maksimovic DJ, Bogdanovic J, Maksimovic V, Nikolic M (2007) Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) Grown at excess manganese. J Plant Nutr Soil Sci 170:739–744
Menzies JG, Ehret DL, Glass ADM, Helmer T, Koch C, Seywerd F (1991) Effects of soluble silicon on the parasitic fitness of Sphaerotheca fuliginea on Cucumis sativus. Phytopathol 81:84–88
Menzies JG, Bowen P, Ehret D, Glass ADM (1992) Foliar applications of potassium silicate reduce severity of powdery mildew on cucumber, muskmelon, and zucchini squash. J Am Soc Hortic Sci 117:902–905
Miyake Y, Takahashi E (1983) Effect of silicon on the growth of solution-cultured cucumber plant. Soil Sci Plant Nutr 29:71–83
Punja ZK, Zhang YY (1993) Plant chitinases and their roles in in resistance to fungal diseases. J nematal 25:4 526–540
Ralph J, Brunow G, Harris PJ, Dixon RA, Schatz PF, Boerjan W (2008) Lignification: are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication? Recent Adv. Polyphenol Res. 36–66
Ratnayake RMRNK, Daundasekera WAM, Ariyarathne HM, Ganehenege MYU (2014) Effect of silicon application on fungal diseases In bitter gourd (Momordica charantia L.) leaves. Proceedings of the PGIS research congress. University of Peradeniya, Sri Lanka p74
Ratnayake RMRNK, Daundasekera WAM, Ariyarathne HM, Ganehenege MYU (2015) Effect of silicon application on downy mildew in bitter gourd (Momordica charantia l.) leaves. Proceedings of the Young Scientists Forum Symposium 2014, National Science and Technology Commission, Sri Lanka. pp163–166
Remus-Borel W, Menzies JG, Belanger RR (2005) Silicon induces antifungal compounds in powdery mildew infected wheat. Physiol and Mol Plant Pathol 66:108–115
Robinson RW, Decker-Walters DS (1996) Crop production science in horticulture 6: Cucurbits CAB International, UK
Rodrigues FA, Vale FXR, Datnoff LE, Prabhu AS, Korndorfer GH (2003) Effect of rice growth stages and silicon on sheath blight development. Phytopathol 93:256–261
Rodrigues FA, Jurick WM, Datnoff LE, Jones JB, Rollins JA (2005) Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol.Mol Plant Pathol 66:144–159
Qin GZ, Tian SP (2005) Enhancement of biocontrol activity of Cryptococcus laurentii by silicon and the possible mechanisms involved. Phytopathol 95:69–75
Samuels AL, Glass ADM, Ehret DL, Menzies JG (1991) Mobility and deposition of silicon in cucumber plants. Plant Cell Environ 14:485–492
Shetty R, Jensen B, Shetty NP, Hansen M, Hansen CW, Starkey KR, Jorgensen HJL (2012) Silicon induced resistance against powdery mildew of rose caused by Podosphaera pannosa. Plant Pathol 61:120–131
Shoemaker HE, Piontek K (1996) On the interaction of lignin peroxidase with lignin. Pure Appl Chem 68(11):2089–2096
Snyder GH (2001) Methods for silicon analysis in plants, soils, and fertilizers. Studies Plant Sci 8:185–196
Torres MA, Jones JDG, Dangle JL (2006) Reactive oxygen species signalling in response to pathogens. Plant Physiol 141:373–378
Trudel J, Asselin A (1989) Detection of chitinase after polyacrylamide gel electrophoresis. Anal biochem 178:362–366
Volk RJ, Kahn RP, Weintraub RL (1958) Silicon content of the rice plant as a factor influencing its resistance to infection by the rice blast fungus, Pyricularia oryzae. Phytopathol 48:179–184
Wang X, Wei Z, Liu D, Zao G (2011) Effects of NaCl and silicon on activities of antioxidative enzymes in roots, shoots and leaves of alfalfa. African J Biotechnol 10(4):545–549
Yang YF, Liang YC, Lou YS (2003) Influences of silicon on peroxidase, superoxide dismutase activity and lignin content of leaves of wheat (Triticum aestivum L.) as related to resistance to powdery mildew. Scientia Agric Sinica 36:813–817
Zeyen, RJ, Carver, TLW, Lyngkjaer, MF (2002) The formation and role of papillae. In: The Powdery Mildews: A Comprehensive Treatise. (eds. Bélanger RR, Bushnell WR, Dik AJ, Carver TLW) The American Phytopathological Society, St. Paul, MN. 107–125
Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M (2006) Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol 175:36–50
Zhu ZJ, Wei GQ, Li T, Qian QQ, JQ Y (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativa L.). Plant Sci 167:527–533
Zou X, Nonogaki H, Welbaum GE (2002) A gel diffusion assay for visualization and quantification of chitinase activity. Mol Biotechnol 22:19–23
Acknowledgments
Financial assistance by the National Research Council of Sri Lanka under the Grant Number NRC 11/118 is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratnayake, R.M.R.N.K., Daundasekera, W.A.M., Ariyarathne, H.M. et al. Some biochemical defense responses enhanced by soluble silicon in bitter gourd-powdery mildew pathosystem. Australasian Plant Pathol. 45, 425–433 (2016). https://doi.org/10.1007/s13313-016-0429-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0429-0