Abstract
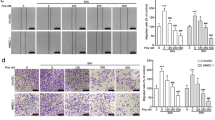
Malignant pleural effusion (MPE) is associated with a poor prognosis in lung cancer. Currently, no effective cure exists for MPE. Chloroquine (CQ) has been demonstrated to induce vascular normalization and inhibit tumor growth. The aim of this study was to assess whether CQ affects MPE. The xenografts mice were divided into normal saline (NS), CQ, or bevacizumab (BE) group. Tumor growth and microvascular density (MVD) were monitored. We explored the effect of CQ on the proliferation, survival, and proangiogenic signaling of tumor cells in vitro. We further evaluated the effects of CQ on the viability, migration, and tube formation of human umbilical vein endothelial cells (HUVECs). A chicken chorioallantoic membrane (CAM) model was used to elucidate the effects of CQ on angiogenesis. Finally, an MPE mouse model were treated by CQ, BE, or NS. The volume of pleural effusion, tumor foci, and MVD was evaluated. CQ therapy group exhibited decreased tumor volume, tumor weight, and MVD in the mouse xenografts. CQ inhibited the proliferation of the tumor cells. However, the expression of vascular endothelial growth factor was not affected. Additionally, CQ inhibited the proliferation, migration, and tube formation of HUVECs and also restrained angiogenesis in the CAM. Western blot showed that CQ might suppress angiogenesis by downregulating p-Akt, Jagged1, and Ang2 in HUVECs. In MPE mice, the volume of the pleural effusion, the number of pleural tumor foci, and the MVD were significantly reduced in the CQ group. Our work demonstrated that CQ played the role of an efficient treatment for MPE.




Similar content being viewed by others
References
Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235–50.
Stathopoulos GT. Translational advances in pleural malignancies. Respirology (Carlton, Vic). 2011;16:53–63.
Haas AR, Sterman DH. Novel intrapleural therapies for malignant diseases. Respiration; International Review of Thoracic Diseases. 2012;83:277–92.
Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med. 2012;186:487–92.
Spella M, Giannou AD, Stathopoulos GT. Switching off malignant pleural effusion formation-fantasy or future? Journal of Thoracic Disease. 2015;7:1009–20.
Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Fidler IJ. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol. 2000;157:1893–903.
Pichelmayer O, Gruenberger B, Zielinski C, Raderer M. Bevacizumab is active in malignant effusion. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO. 2006;17:1853.
Massarelli E, Onn A, Marom EM, Alden CM, Liu DD, Tran HT, Mino B, Wistuba II, Faiz SA, Bashoura L, Eapen GA, Morice RC, Jack Lee J, Hong WK, Herbst RS, Jimenez CA. Vandetanib and indwelling pleural catheter for non-small-cell lung cancer with recurrent malignant pleural effusion. Clinical Lung Cancer. 2014;15:379–86.
Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, ph and the anti-malarial action of chloroquine. Nature. 1972;235:50–2.
Zhang Y, Liao Z, Zhang LJ, Xiao HT. The utility of chloroquine in cancer therapy. Curr Med Res Opin. 2015;31:1009–13.
Vezmar M, Georges E. Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem Pharmacol. 2000;59:1245–52.
Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–90.
Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–33.
Kim EL, Wustenberg R, Rubsam A, Schmitz-Salue C, Warnecke G, Bucker EM, Pettkus N, Speidel D, Rohde V, Schulz-Schaeffer W, Deppert W, Giese A. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-Oncology. 2010;12:389–400.
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34.
Lesiak A, Narbutt J, Kobos J, Kordek R, Sysa-Jedrzejowska A, Norval M, Wozniacka A. Systematic administration of chloroquine in discoid lupus erythematosus reduces skin lesions via inhibition of angiogenesis. Clin Exp Dermatol. 2009;34:570–5.
Grimaldi A, Balestrieri ML, D’Onofrio N, Di Domenico G, Nocera C, Lamberti M, Tonini G, Zoccoli A, Santini D, Caraglia M, Pantano F. The synergistic effect of everolimus and chloroquine on endothelial cell number reduction is paralleled by increased apoptosis and reduced autophagy occurrence. PLoS One. 2013;8:e79658.
Potvin F, Petitclerc E, Marceau F, Poubelle PE. Mechanisms of action of antimalarials in inflammation: induction of apoptosis in human endothelial cells. Journal of Immunology (Baltimore, Md: 1950). 1997;158:1872–9.
Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion AC, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquiere B, Annaert W, Agostinis P, Carmeliet P. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26:190–206.
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87.
Takanami I. Overexpression of Ang-2 mRNA in non-small cell lung cancer: association with angiogenesis and poor prognosis. Oncol Rep. 2004;12:849–53.
Bose D, Meric-Bernstam F, Hofstetter W, Reardon DA, Flaherty KT, Ellis LM. Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. The Lancet Oncology. 2010;11:373–82.
Hajitou A, Grignet C, Devy L, Berndt S, Blacher S, Deroanne CF, Bajou K, Fong T, Chiang Y, Foidart JM, Noel A. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2002;16:1802–4.
Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22.
Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nature Reviews Cancer. 2008;8:309–16.
YL H, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, Aghi MK. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72:1773–83.
Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol. 2010;22:177–80.
Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81.
Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–27.
Giannou AD, Marazioti A, Spella M, Kanellakis NI, Apostolopoulou H, Psallidas I, Prijovich ZM, Vreka M, Zazara DE, Lilis I, Papaleonidopoulos V, Kairi CA, Patmanidi AL, Giopanou I, Spiropoulou N, Harokopos V, Aidinis V, Spyratos D, Teliousi S, Papadaki H, Taraviras S, Snyder LA, Eickelberg O, Kardamakis D, Iwakura Y, Feyerabend TB, Rodewald HR, Kalomenidis I, Blackwell TS, Agalioti T, Stathopoulos GT. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125:2317–34.
Lin H, Tong ZH, Xu QQ, Wu XZ, Wang XJ, Jin XG, Ma WL, Cheng X, Zhou Q, Shi HZ. Interplay of th1 and th17 cells in murine models of malignant pleural effusion. Am J Respir Crit Care Med. 2014;189:697–706.
Acknowledgments
This study was supported by the Natural Science Foundation of Jiangsu Province, China (No. BK20140736), the Standard Diagnosis and Treatment Program of Key Disease in Jiangsu Province (No. BL2013026), and the National Natural Science Foundation of China (No. 81370172).
Author contributions
Qian Li participated in the experimental design and carried out animal surgeries; Li-hong Ma worked on the histological and immunohistochemistry staining, analyzed the data, and drafted the manuscript. Chen-hui Ma contributed to conception of the study, experimental design, analyzed the data and drafted the manuscript. Dong-mei Yuan helped with the animal surgeries, histological staining, and drafting of the manuscript. Tang-feng Lv contributed to the study’s conception, analyzed the data, and revised the manuscript. Ya-fang Liu participated in designing the experiment and revising the manuscript. Yong Song contributed to conception of the study, experimental design, analyzed the data, and revised the manuscript. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were conducted in accordance with the guidelines approved by the Institute Animal Care and Use Committee of Jinling Hospital.
Conflicts of interest
None.
Additional information
Qian Li and Dong-mei Yuan contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Li, Q., Yuan, Dm., Ma, Lh. et al. Chloroquine inhibits tumor growth and angiogenesis in malignant pleural effusion. Tumor Biol. 37, 16249–16258 (2016). https://doi.org/10.1007/s13277-016-5441-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5441-z