Abstract
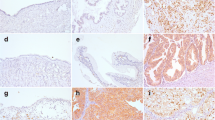
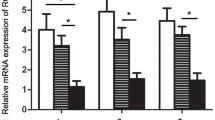
We aimed to investigate the expression of FOXP1 in ovarian tumors and correlate it with clinicopathological parameters, chemotherapy resistance, and prognosis. FOXP1 messenger RNA (mRNA) expression was examined in fresh ovarian cancer tissues and normal ovarian tissues, and FOXP1 protein expression was determined in a total of 201 ovarian tissue samples, including 152 cases of primary epithelial ovarian cancer, 26 borderline ovarian tumors, 13 benign ovarian tumors, and 10 normal ovarian tissues. Complete chemotherapy and follow-up data were available in 92 of the 152 epithelial ovarian cancer patients. The relationship between FOXP1 protein expression and ovarian cancer pathological characteristics, chemotherapy resistance, and survival time was analyzed. FOXP1 mRNA expression was downregulated in ovarian cancer tissues compared with that in normal ovarian tissues. Decreased nuclear and increased cytoplasmic FOXP1 protein expression was correlated with increasing tumor grade. Nuclear FOXP1 expression was an independent risk factor associated with chemotherapy resistance and the prognosis of patients with ovarian cancer. FOXP1 expression is closely related to the degree of malignancy of epithelial ovarian cancer and may be a reliable index of the chemoresistance and prognosis of ovarian cancer.




Similar content being viewed by others
References
Hu H, Wang B, Borde M, Nardone J, Maika S, Allred L, et al. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819–26.
Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, et al. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–87.
Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, et al. Down-regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood. 2008;112:6699–711.
Li S, Wang Y, Zhang Y, Lu MM, DeMayo FJ, Dekker JD, et al. Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development. 2012;139:2500–9.
Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:2490–7.
Banham AH, Connors JM, Browen PJ, Cordell JL, Ott G, Sreenivasan G, et al. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11:1065–72.
Pronina IV, Loginov VI, Kholdyrev DS, Kazubskaia TP, Braga EA. Alterations of expression level of RASSFIA gene in primary epithelia tumors of various locations. Mol Biol (Mosk). 2012;46:260–8.
Palumbo E, Tosoni E, Matricardi L, Russo A. Genetic instability of the tumor suppressor gene FHIT in normal human cells. Genes Chromosomes Cancer. 2013;52:832–44.
Banham AH, Beasley N, Campo E, Fernandez PL, Fidler C, Gatter K, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820–9.
Shigekawa T, Ijichi N, Ikeda K, Horie-Inoue K, Shimizu C, Saji S, et al. FOXP1, an eatrogen-inducible transcription factor, modulates cell proliferation in breast cancer cells and 5-year recurrence-free survival of patients with tamoxifen-treated breast cancer. Horm Cancer. 2011;2:286–97.
Takayama K, Horie-Inoue K, Ikeda K, Urano T, Murakami K, Hayashizaki Y, et al. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem Biophys Res Commun. 2008;374:388–93.
Kim YS, Hwan JD, Bae S, Bae DH, Shick WA. Identification of differentially expressed genes using an annealing control primer system in stage III serous ovarian carcinoma. BMC Cancer. 2010;10:576.
Chien WH, Gau SS, Chen CH, Tsai WC, Wu YY, Chen PH, et al. Increased gene expression of FOXP1 in patients with autism spectrum disorders. Mol Autism. 2013;4:23.
Fox SB, Brown P, Han C, Ashe S, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinoma. Clin Cancer Res. 2004;10:3521–7.
Bates GJ, Fox SB, Han C, Launchbury R, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor-beta in primary invasive breast carcinomas. Breast Cancer Res Treat. 2008;111:453–9.
Giatromanolaki A, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, Banham AH. Loss of expression and nuclear/cytoplasmic localization of the FOXP1 foxkhead transcription factor are common events in early endometrial cancer: relationship with estrogen receptors and HIF-1a expression. Mod Pathol. 2006;19:9–16.
Toma MI, Grosser M, Herr A, Aust DE, Meye A, Hoefling C, et al. Loss of heterozygosity and copy number abnormality in clear cell renal cell carcinoma discovered by high-density affymetrix 10K single nucleotide polymorphism mapping array. Neoplasia. 2008;10:634–42.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82.
Rayoo M, Yan M, Takano EA, Bates GJ, Brown PJ, Banham AH, et al. Expression of the forkhead box transcription factor FOXP1 is associated with oestrogen receptor alpha, oestrogen receptor beta and improved survival in familial breast cancers. J Clin Pathol. 2009;62:896–902.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement table 1
(DOC 30 kb)
Supplement table 2
(DOC 54 kb)
Rights and permissions
About this article
Cite this article
Hu, Z., Zhu, L., Gao, J. et al. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumor Biol. 36, 7269–7275 (2015). https://doi.org/10.1007/s13277-015-3383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3383-5