Abstract
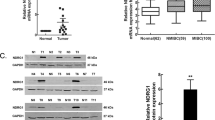
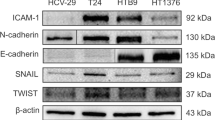
NOV is pro-tumourigenic via epithelial-mesenchymal transition (EMT) in several malignancies but is not studied in bladder cancer (BCa). Whether NOV is responsible for bladder carcinogenesis and the underlying mechanism is unclear. Using immunohistochemical staining, we quantified expressions of NOV, pS6, Vimentin and E-cadherin in 66 bladder cancer and 10 normal bladder urothelium samples. EMT was profiled by EMT index (EMTi) calculated as the ratio of Vimentin to E-cadherin. In vitro and in vivo studies were carried out to profile the role of NOV in the tumourigenesis of BCa. NOV was upregulated in bladder cancer compared to normal tissue, and its expression was correlated to pS6 and EMTi. Expression of NOV was higher in recurrent and multiple tumours and was increased with progression of tumour grade. NOV expression was also higher in BCa cell lines. Silence of NOV attenuated EMT, decreased invasion and migration of BCa cells. Silence of NOV also inhibited xenograft tumour growth and decreased tumour EMT. NOV is pro-tumourigenic in bladder cancer especially in nonmuscle-invasive entities (NMIBC). NOV may promote carcinogenesis via promotion of EMT and association with increased mTOR activity.



Similar content being viewed by others
References
Feng C, Guan M, Ding Q, Zhang Y, Jiang H, et al. Expression of pigment epithelium-derived factor in bladder tumour is correlated with interleukin-8 yet not with interleukin-1α. J Huazhong Univ Sci Technol [Med Sci]. 2011;31:21–5.
Ferley J, Bray F, Pisani P, Parkin DM. Globalcan 2000: cancer incidence, mortality and prevalence world- wide, version 1.0 LARC Cancer Base No.5. Lyon: IARC Press; 2001.
Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–5. discussion 475-467.
Messing EM. Urothelial tumors of the urinary tract. In: Walsh PC, Retik AB, Vaughan Jr ED, Wein AJ, editors. Campbell’s urology. 8th ed. Philadelphia: Saunders; 2002. p. 2750–1.
Messing EM. Urothelial tumors of the bladder. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh's urology. 9th ed. Philadelphia: Saunders; 2007. p. 17–2430.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Yun SJ, Kim WJ. Role of the epithelial-mesenchymal transition in bladder cancer: from prognosis to therapeutic target. Korean J Urol. 2013;54:645–50.
Kleer CG, Zhang Y, Pan Q, Merajver SD. WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates IGF signaling in inflammatory breast cancer. Neoplasia. 2004;6:179–85.
Yu C, Le AT, Yeger H, Perbal B, Alman BA. NOV (CCN3) regulation in the growth plate and CCN family member expression in cartilage neoplasia. J Pathol. 2003;201:609–15.
Cui L, Xie R, Dang S, Zhang Q, Mao S, et al. NOV promoted the growth and migration of pancreatic cancer cells. Tumour Biol. 2013. doi:10.1007/s13277-013-1418-3.
Seront E, Pinto A, Bouzin C, Bertrand L, Machiels JP, et al. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–92.
Feng C, Wang P, Guan M, Jiang H, Wen H, et al. Urinary BLCA-4 is highly specific for detection of bladder cancer in Chinese Han population and is related to tumour invasiveness. Folia Biol (Praha). 2011;57:242–7.
Feng C, Wu Z, Guo T, Jiang H, Guan M, et al. BLCA-4 expression is related to MMP-9, VEGF, IL-1α and IL-8 in bladder cancer but not to PEDF, TNF-α or angiogenesis. Pathol Biol. 2012;60:e36–40.
Feng C-C, Ding Q, Zhang Y-F, Jiang H-W, Wen H, et al. Pigment epithelium-derived factor expression is down-regulated in bladder tumors and correlates with vascular endothelial growth factor and matrix metalloproteinase-9. Int Urol Nephrol. 2011;43:383–90.
Feng C-C, Wang P-H, Ding Q, Guan M, Zhang Y-F, et al. Expression of pigment epithelium-derived factor and tumor necrosis factor-α is correlated in bladder tumor and is related to tumor angiogenesis. Urol Oncol Semin Orig Investig. 2013;31:241–6.
Mikaelian I, Malek M, Gadet R, Viallet J, Garcia A, et al. Genetic and pharmacologic inhibition of mTORC1 promotes EMT by a TGF-beta-independent mechanism. Cancer Res. 2013;73:6621–31.
Leal P, Garcia P, Sandoval A, Buchegger K, Weber H, et al. AKT/mTOR substrate P70S6K is frequently phosphorylated in gallbladder cancer tissue and cell lines. Onco Targets Ther. 2013;6:1373–84.
Maillard M, Cadot B, Ball RY, Sethia K, Edwards DR, et al. Differential expression of the ccn3 (nov) proto-oncogene in human prostate cell lines and tissues. Mol Pathol. 2001;54:275–80.
Ding G, Feng C, Jiang H, Ding Q, Zhang L, et al. Combination of rapamycin, CI-1040, and 17-AAG inhibits metastatic capacity of prostate cancer via Slug inhibition. PLoS One. 2013;8:e77400.
Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56.
Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–51.
Wallin JJ, Guan J, Edgar KA, Zhou W, Francis R, et al. Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes. PLoS One. 2012;7:e36402.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jie Chen and Yi Gao contributed equally.
Rights and permissions
About this article
Cite this article
Chen, J., Gao, Y., Xu, B. et al. NOV is upregulated and promotes migration and invasion in bladder cancer. Tumor Biol. 35, 6749–6755 (2014). https://doi.org/10.1007/s13277-014-1919-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1919-8