Abstract
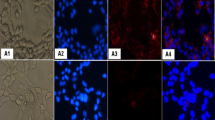
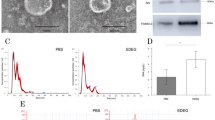
A proteomic-based approach was used to search for potential markers in the plasma of hamsters in which cholangiocarcinoma (CCA) was induced by Opisthorchis viverrini infection and N-nitrosodimethylamine treatment. The plasma proteins of CCA-induced hamsters were resolved by 1-D PAGE, digested by trypsin, and analyzed by LC-MS/MS. From the criteria of protein ID scores >15 and an overexpression of at least three times across all time points, 37 proteins were selected. These overexpressed proteins largely consisted of signal transduction, structural, transport, and transcriptional proteins in the order. Among the most frequently upregulated proteins, exostosin 1 (EXT1) was selected for further validation. By western blot analysis, the EXT1 expression level in the plasma of hamster CCA was significantly higher than that of controls at 1 month and thereafter. Immunohistochemistry revealed that EXT1 was expressed at vascular walls and fibroblasts at 21 days (before tumor onset) and at 2 months (early CCA) posttreatment. Its expression was also observed in bile duct cancer cells during tumor progression at 6 months posttreatment. In the human CCA tissue microarray, EXT1 immunoreactivity was found not only in vascular walls and fibroblasts but also in bile duct cancer cells and was positive in 89.7 % (61/68) of the cases. By ELISA and immunoblotting, plasma EXT1 level was significantly higher in human CCA compared to healthy controls. In conclusion, these results suggest that increased expression of EXT1 level in the plasma might be involved in CCA genesis and might be a potential biomarker of CCA.






Similar content being viewed by others
Abbreviations
- OV:
-
Opisthorchis viverrini
- CCA:
-
Cholangiocarcinoma
- EXT1:
-
Exostosin 1
- ECM:
-
Extracellular matrix
References
Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85.
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14.
Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, northeast thailand. Trop Med Int Health. 2004;9:588–94.
Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from thailand. Curr Opin Gastroenterol. 2008;24:349–56.
Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200.
Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279–84.
Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected syrian golden hamsters. Cancer Res. 1978;38:4634–9.
Prakobwong S, Yongvanit P, Hiraku Y, Pairojkul C, Sithithaworn P, Pinlaor P, et al. Involvement of mmp-9 in peribiliary fibrosis and cholangiocarcinogenesis via rac1-dependent DNA damage in a hamster model. Int J Cancer. 2010;127:2576–87.
Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50:1273–81.
Juan HF, Chen JH, Hsu WT, Huang SC, Chen ST, Yi-Chung Lin J, et al. Identification of tumor-associated plasma biomarkers using proteomic techniques: from mouse to human. Proteomics. 2004;4:2766–75.
Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67.
Khoontawad J, Laothong U, Roytrakul S, Pinlaor P, Mulvenna J, Wongkham C, et al. Proteomic identification of plasma protein tyrosine phosphatase alpha and fibronectin associated with liver fluke, Opisthorchis viverrini, infection. PLoS One. 2012;7:e45460.
Guo J, Wang W, Liao P, Lou W, Ji Y, Zhang C, et al. Identification of serum biomarkers for pancreatic adenocarcinoma by proteomic analysis. Cancer Sci. 2009;100:2292–301.
Yonglitthipagon P, Pairojkul C, Chamgramol Y, Mulvenna J, Sripa B. Up-regulation of annexin a2 in cholangiocarcinoma caused by Opisthorchis viverrini and its implication as a prognostic marker. Int J Parasitol. 2010;40:1203–12.
Lin X, Gan L, Klein WH, Wells D. Expression and functional analysis of mouse ext1, a homolog of the human multiple exostoses type 1 gene. Biochem Biophys Res Commun. 1998;248:738–43.
Lin X, Wells D. Isolation of the mouse cdna homologous to the human ext1 gene responsible for hereditary multiple exostoses. DNA Seq. 1997;7:199–202.
de Andrea CE, Hogendoorn PC. Epiphyseal growth plate and secondary peripheral chondrosarcoma: the neighbours matter. J Pathol. 2012;226:219–28.
Cook A, Raskind W, Blanton SH, Pauli RM, Gregg RG, Francomano CA, et al. Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet. 1993;53:71–9.
Bernard MA, Hall CE, Hogue DA, Cole WG, Scott A, Snuggs MB, et al. Diminished levels of the putative tumor suppressor proteins ext1 and ext2 in exostosis chondrocytes. Cell Motil Cytoskeleton. 2001;48:149–62.
Osterholm C, Barczyk MM, Busse M, Gronning M, Reed RK, Kusche-Gullberg M. Mutation in the heparan sulfate biosynthesis enzyme ext1 influences growth factor signaling and fibroblast interactions with the extracellular matrix. J Biol Chem. 2009;284:34935–43.
Zak BM, Crawford BE, Esko JD. Hereditary multiple exostoses and heparan sulfate polymerization. Biochim Biophys Acta. 2002;1573:346–55.
Hennekam RC. Hereditary multiple exostoses. J Med Genet. 1991;28:262–6.
Schmale GA, Conrad 3rd EU, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76:986–92.
Jhandier MN, Kruglov EA, Lavoie EG, Sevigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of ntpdase2. J Biol Chem. 2005;280:22986–92.
Juttijudata P, Chiemchaisri C, Palavatana C, Churnratanakul S. Causes of cholestasis in Thailand. A study of 276 consecutive patients. Am J Surg. 1984;147:360–6.
Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, DePinho RA. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622–7.
Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–3.
Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54.
McCormick C, Leduc Y, Martindale D, Mattison K, Esford LE, Dyer AP, et al. The putative tumour suppressor ext1 alters the expression of cell-surface heparan sulfate. Nat Genet. 1998;19:158–61.
Lind T, Tufaro F, McCormick C, Lindahl U, Lidholt K. The putative tumor suppressors ext1 and ext2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J Biol Chem. 1998;273:26265–8.
Tatrai P, Egedi K, Somoracz A, van Kuppevelt TH, Ten Dam G, Lyon M, et al. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J Histochem Cytochem. 2010;58:429–41.
Koo CY, Sen YP, Bay BH, Yip GW. Targeting heparan sulfate proteoglycans in breast cancer treatment. Recent Pat Anticancer Drug Discov. 2008;3:151–8.
Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2004;10:5178–86.
Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–6.
Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50.
Acknowledgments
This work was supported by The Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (to JK and SP), Thailand Research Fund (RMU5380016), a Research Team Strengthening Grant from BIOTEC-NSTDA, Thailand, The Invitation Research from the Faculty of Medicine, Khon Kaen University, Thailand, and The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University, Thailand. We thank a Research Assistant, Faculty of Medicine, Khon Kaen University, Thailand, for the technical support. We wish to acknowledge the support of the Khon Kaen University Publication Clinic, Research and Technology Transfer Affairs, Khon Kaen University, for their assistance. We also thank Prof. Yukifumi Nawa, Research Affairs, Faculty of Medicine, Khon Kaen University, Thailand, for his suggestion and critical reading of the manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoontawad, J., Hongsrichan, N., Chamgramol, Y. et al. Increase of exostosin 1 in plasma as a potential biomarker for opisthorchiasis-associated cholangiocarcinoma. Tumor Biol. 35, 1029–1039 (2014). https://doi.org/10.1007/s13277-013-1137-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1137-9