Abstract
FeCl3 coagulation was used to achieve maximum reduction of COD, phosphate, sulfate and color and process optimization (FeCl3 dosage, pH, reaction time) was done by BBD-RSM. Responses were recorded in terms of EC, COD, phosphate, color and sulfate removal. Variables A (pH) and B (reaction time) were negatively related to removal of COD and phosphate, whereas, C (FeCl3 dosage) was positive in case of COD removal and negative for phosphate removal. pH and coagulant dosage had negative relationship with color removal; however, reaction time showed positive relationship. In case of percent sulfate removal, variable A (pH) demonstrated negative relationship whereas B (reaction time) and C (FeCl3 dosage) were found to be positively related. Numerical optimization of the model revealed a maximum reduction of 71, 93, 86 and 99.6 % COD, phosphate, color and sulfate at optimal FeCl3 dosage = 3 g/l, pH 8, and reaction time = 95 min.
Similar content being viewed by others
Introduction
Growing urbanization and rapid industrialization have further worsened the problem of solid waste management in almost each and every part of the world. Approximately 95 % of solid waste generated worldwide is disposed off in landfills (Kurniawan et al. 2006). Percolation of rainwater and/or the moisture content of the waste itself gives rise to the generation of dark-colored concentrated mixture that leaches from the waste in the form of ‘garbage juice’ (Sari et al. 2013; Zhao et al. 2013a, b). On an average, 1 ton of MSW in a landfill site produces 0.2 m3 of leachate (Kurniawan and Lo 2009). Landfill leachate is characterized by the presence of high pH, suspended solids (SS), chemical oxygen demand (COD), color and alkalinity which can significantly contaminate the receiving water bodies (Kjeldsen et al. 2002; Adlan et al. 2011; Zhao et al. 2013a, b; De Torres-Socías et al. 2014). Therefore, landfill leachate treatment has always been a subject of considerable interest (Oller et al. 2011; Sari et al. 2013).
On the basis of chemical constituents and the ambient conditions, several physical, chemical and biological methods are being employed solely and/or in integration for the removal of unwanted constituents from landfill leachate (Castrillón et al. 2010; Xiao et al. 2013; Zhao et al. 2013a, b; Anfruns et al. 2013; Wang et al. 2014a, b). The presence of refractory compounds and large variations in type and concentration of pollutants render biological methods inapplicable for the purpose in spite of being cost-effective, reliable, simple and economical (Kurniawan et al. 2006; Abbas et al. 2009; Wang et al. 2012; Wu et al. 2014). For old, stabilizing leachates mostly physico-chemical techniques are recommended (Ghafari et al. 2010).
Coagulation/flocculation is a widely employed and oldest treatment methods in landfill leachate treatment (Nair and Ahammed 2013; Kamaruddin et al. 2014). Selection of the coagulant type and dosages critically determine the success of as well as efficiency of treatment achieved. Generally, coagulation for landfill leachate treatment involves the addition of ferric/alum salts (Ayoub et al. 2001). However, it has been established that iron salts are more efficient than aluminium ones (Renou et al. 2008). Therefore, in the present study we have chosen FeCl3 as a coagulant. Other factors which affect the efficiency of coagulation and hence need optimization are pH, mixing, temperature and retention time etc. (Wang et al. 2007).
Conventionally, multi-factor optimization is done by one-factor at-a-time approach while keeping the others constant at a specific set of conditions. It is easy to design and implement but one-factor at a time approach is time-consuming and do not take into account the interactions between factors. Consequently, it cannot be used to find the optimum conditions with greater accuracy (Wang et al. 2014a, b). To overcome these limitations, statistical experimental designs [including response surface methodology (RSM)] were proposed. Applicability of RSM lies in the measurement of responses for multiple variables influencing the system (Jin et al. 2014). RSM together with Box–Behnken design (RSM-BBD) was chosen for the current study to quantitatively investigate the effects of different operating conditions and their interactions on landfill leachate treatment efficiency while using FeCl3 as a coagulant. The main objective of this work was to optimize the coagulation process by BBD of RSM. Percent reduction in COD, color, phosphate and sulfate and EC were chosen as the dependent output variables. FeCl3 dosage, initial pH and reaction time were chosen as the influence factors. Generally phosphate and sulfate removal are not studied as responses while treating landfill leachate. However, as now-a-days combined and sequential processes are increasingly being employed for the treatment of landfill leachate employing physicochemical pre or post treatments. While using FeCl3 coagulation as a pre-treatment method followed by any biological treatment; we are also concerned with the concentration of phosphate and sulfate present in the wastewater and an external supply is needed to maintain the required concentrations. EC was chosen as a response to observe the variation on the electrical conductivity of the resulting effluent and can be a useful parameter in electrochemical treatments as well as novel treatment methods such as MFC.
Materials and methods
Sample collection and characterization
The leachate samples were collected from a landfill site located in Bhalaswa, NW Delhi, India (28°44′26″N 77°9′26″E). The site has been operational since 1993. The site occupies 21.06 acres of land and receives about 2200 tons of waste per day (Mor et al. 2006). 50 l leachate samples were obtained from a nearby drain to which all the leachate flows and were stored at 4 °C before being used and analyzed. All samples used in the present work were collected from a single point source at single site on different times. However, general trend is a subject of subsequent papers and beyond the scope of the present work.
Experimental methodology
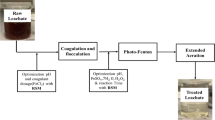
Coagulation experiments were carried out in seventeen 250 ml Erlenmeyer flasks. Each flask was filled with 100 ml of the raw leachate and was continuously mixed at 140 rpm in an orbital shaker. Stepwise coagulation process was carried out in the following manner: (1) 100 ml leachate sample was taken in a flask (250 ml); (2) pre-decided quantities of solid FeCl3·6H2O were added to the flasks in a single step (2, 2.5 and 3 g/l); (3) its pH was adjusted (to 8, 10 and 12) in accordance with the experimental design using NaOH after adding the coagulant; (4) the mixture was stirred for the specified time (30, 90 and 150 min) at 140 rpm velocity; (5) allowed to settle for 1 h; (6)and supernatant was analyzed for COD, color, phosphate, EC, TDS and Salt concentrations and percent removal were calculated where needed. All the runs were carried out at room temperature (Table 1).
Experimental design and data analysis
Box–Behnken design has specific positioning of design points and always has three levels for each factor coded as −1, 0, and +1. It has been created for estimating a quadratic model and provides strong coefficient estimates near the center of the design space (where the presumed optimum is), but weaker at the corners of the cube (where there are no design points). It is a rotatable quadratic design where factor combinations are at the midpoints of the edges and at the centre (Bezerra et al. 2008; Ferreira et al. 2007). A Box–Behnken statistical experimental design with the RSM was used to investigate the effects of the three independent variables namely FeCl3 dosage, initial pH and reaction time on coagulation. % COD, phosphate, sulfate and color removals were chosen as the response parameters. EC, TDS and Salt concentrations were also observed in the resulting effluent. BBD was chosen because of its economical and efficient nature. Table 2 presents the details of operating conditions for coagulation process. To evaluate the contribution of the three variables, experimental data were analyzed and fitted to the following second-order polynomial model using Design Expert 9.0 software:
where Y represents the predicted response; Xi and Xj are the independent variables, β 0, βi, βii and βij are regression coefficients for intercept, linear, quadratic and interaction coefficients respectively, ε is the error and k is the number of variables studied (Nair and Ahammed 2013). The significance of the model equation and model terms was evaluated by F test (Jadhav et al. 2013). The quality of the quadratic model equation was expressed by the determination coefficient R 2 and adjusted R 2 (Jadhav et al. 2013). Analysis of variance (ANOVA) was applied to evaluate the statistical significance of the model (Jadhav et al. 2013). The optimal values were obtained by solving the regression equation and contour plots were used for analyzing the interactive effect of each variable (Jadhav et al. 2013, 2014).
Analytical methods
The COD of the samples were analyzed in accordance with the Standard Methods using the closed reflux titrimetric method (APHA 1998). pH, TDS, Salt concentrations and EC were measured digitally by multi response meter after proper calibration (Eutech pH meter Model PC-510 from Eutech Instruments). Phosphate was measured with the ammonium molybdate method using a UV visible spectrophotometer (at 690 nm). Color was also measured using UV–Vis spectrophotometer (390 nm). Sulfate was measured with barium chloride method taking absorbance at 420 nm. All experiments were done at room temperature and were repeated at least thrice to ensure statistical accuracy.
Calculations
Percent removals of the responses (COD, phosphate, color and sulfate) from leachate were calculated as follows:
where C i and C f are the initial and final concentrations of the respective parameters.
Results and discussion
Validation of response surface models and statistical analysis
The physicochemical characteristics of the raw leachate are summarized in Table 1. A total of 17 set of experiments were run according to BBD. BBD matrix for experimental design (real and coded values of the three factors, i.e. pH, coagulant dosage and reaction time) and observed and predicted responses for percent removal of COD, phosphate, color and sulfate have been summarized in Table 3. Second-order polynomial equations were used to draw relationship between independent variables and responses. The regression equation coefficients were calculated and data was fitted to a second-order polynomial equation for removal of COD, phosphate, color and sulfate from landfill leachate using FeCl3 as a coagulant.
As is obvious from the results, the obtained values were reasonably close to the predicted experimental values, specifying the efficiency of developed model to describing the correlation between the controlling parameters on treatment efficiency of landfill leachate. Within the chosen range of the experiment, observed percent removal efficiencies varied between 57.4 and 71.3 % for COD (average being 64.38); 87–94 % for phosphate (average 90 %); 73–86 % for color (average 79) and 92–99.6 % (average 97.7) for sulfate. Table 4 provides the ANOVA of variables fitted to quadratic polynomial models as well as other statistical parameters for COD, phosphate, color and sulfate removals and EC.
A closer analysis of the table reveals that all the models were significant at the 5 % confidence level and in most of the cases P values were less than 0.05. Values obtained for correlation coefficient (R 2 = 0.9820–0.9980) in the present study for COD, Phosphate, color, sulfate and EC were higher than 0.80. Correlation coefficient needs to be at a minimum of 0.80 for a good fit of model (Abu Amr et al. 2014). A high R 2 value (closer to 1) demonstrates good accordance between the calculated and observed results within the range of experiment and shows that a desirable and reasonable agreement with adjusted R 2 is necessary (Nordin et al. 2004; Abu Amr et al. 2014). The range in predicted response relative to its associated error can be measured by adequate precision. The “Adequate Precision” ratio should be higher than 4 so that the predicted models can be used to navigate the space (Kousha et al. 2012). For the present model, AP values models lie between 20.13 and 67.91; which is an adequate signal for the model and suggests that the data obtained through predicted quadratic model is reliable and can be used to navigate the design space. Based on the results, the response surface models constructed for predicting percent COD, phosphate, color, sulfate removal efficiency and EC concentrations were considered reasonable.
Optimization of variables for COD, phosphate, color and sulfate removal
The effect of factors, i.e. pH, reaction time and coagulant dosage on the responses, i.e. percent removal of COD, phosphate, color and sulfate was analyzed on the basis of quadratic polynomial equations derived on the basis of RSM (Eqs. 3–6). A (pH) was a vital factor (P > 0.0001) and had a negative effect on all the responses, i.e. percent removal of COD, phosphate, color and sulfate (Eqs. 3–6) from landfill leachate thus suggesting decreased removal with increase in pH. B, reaction time (min) (P > 0.0001) considerably affects the removal efficiency of the selected parameters by determining the extent of completion of the reaction. Independent variable time had a negative effect on COD and phosphate (Eqs. 3, 4) and positive effect on color and sulfate removal (Eqs. 5, 6). C, coagulant dosage (P > 0.0001) has considerable effect on the removal efficiency and plays an important role in enhanced removal. Within the chosen range, coagulant dose had positive effect on COD and sulfate removal and negative effect on phosphate and color removal (Eqs. 3–6). COD and sulfate removal increased as the initial concentration of coagulant increased because at higher concentration more coagulant molecules or ions (Fe2+ and Cl−) were available for binding with the impurities thus resulting in better removal. However, phosphate and color removal efficiencies decreased with increase in coagulant dosage. As seen from the equations; elevated parameter estimation values of variables A, B, C, AB, AC, BC, A2, B2, C2 demonstrated high significance and hence summit the magnitude of these variables in the coagulation process. The variables A (pH) and B (reaction time) were negatively related to removal of COD and phosphate, whereas C (FeCl3 dosage) was positive in case of COD removal and negative for phosphate removal. pH and coagulant dosage had negative relationship with color removal; however, reaction time showed positive relationship. In case of percent sulfate removal, variable A (pH) demonstrated negative relationship whereas B (reaction time) and C (FeCl3 dosage) gave us an idea about its positive relationship.
Percent COD removal
The Model F value of 42.33 implies the model is significant. Values of “Prob > F” <0.0500 indicate model terms are significant. In this case A, B, C, AC, BC, A2, B2 and C2 were significant model terms. Values >0.1000 indicate the model terms are not significant. The “Lack of Fit F value” of 0.24 implies the Lack of Fit is not significant relative to the pure error. Non-significant lack of fit is good and we want the model to fit. The “Pred R 2” of 0.9320 is in reasonable agreement with the “Adj R 2” of 0.9588, i.e. the difference is <0.2.
The interaction of two factors while keeping the third one constant on the percent removal efficiency of COD, phosphate, color and sulfate has been illustrated in 3D response surface plots (Figs. 2, 3, 4, 5a–c). Figure 2a–c demonstrates the interactive effect of two variables, i.e. (a) reaction time and pH at constant coagulant dose of 2.5 g/l, (b) pH and coagulant dose at constant reaction time of 90 min, and (c) % COD removal and reaction time at constant reaction pH (10) on percent COD removal efficiency. In Fig. 1a, COD removal increased with increase of pH from 8.0 to 10.0 and reaction time from 30 to 90 min and the exhibited a decreasing trend with any further increase in pH and time. COD removal was highest (68 %) around a pH of 8, reaction time around 90 min at a constant coagulant dosage of 2.5 g/l. COD removal increased with both, i.e. coagulant dosage as well as pH. A Maximum removal of 71.21 % COD was observed at a dosage of 3 g/l, pH 8.2 and reaction time 90 min (Fig. 1b). In a recent study, Liu et al. (2012) reported an optimum pH of 8 and dose 10 g/l for 68.65 % reduction in COD. It was observed that coagulant dosage had a far significant effect on COD removal within the chosen range as compared to that of pH and reaction time. At low concentration of FeCl3, COD removal was found to be lower (60 % at 2 g/l and pH 8) which might be due to non availability of the coagulant to neutralize all the impurities present. Higher coagulant dosage had enough species to react with all of the impurities. COD removal also increased with increase in pH from 60 to approximately 64.8 % around a pH value of 10.7, coagulant dose of 2 g/l and constant reaction time of 90 min. In Fig. 1c, at a constant pH of 10, COD removal increased with increasing concentration of FeCl3 and reaction time. A maximum removal of around 70 % was observed at 3 g/l coagulation dose, pH 10 after 58 min of the reaction. Adlan et al. (2011) combined FeCl3 coagulation and dissolved air flotation (DAF) for the maximum removal of COD and color from semiaerobic landfill leachate using CCD of RSM and reported respective removal efficiencies of 75 and 93 %. Boumechhour et al. (2013) reported 64.18 % COD removal at the optimum dosage of 1.2 g/l of FeCl3 at pH 6. Moradi and Ghanbari (2014) using RSM, employed ferric chloride coagulation as a pre-treatment process for landfill leachate treatment prior to Fenton oxidation and achieved approximately 65, 79 and 95 % removals of COD, color and TSS respectively (pH 7 and 1500 mg/l FeCl3). Rivas et al. (2004) combined a sequential coagulation–flocculation and Fenton oxidation process in their study to achieve maximum removal of colloidal particles present in the leachate. About 90 % COD removal was achieved with an initial concentration of 7400 mg/l at pH 8.5 by using 0.8 g/l of FeCl3. Amokrane et al. (1997) reported that FeCl3 when used as coagulant was able to remove 55 % COD. A combination of coagulation and Fenton oxidation with FeCl3 as a coagulant reduced COD by up to 90 % (US Environmental Protection Agency 2002).
Percent phosphate removal
The Model F value of 59.45 implies the model is significant. In this case A, B, A2, B2, C2 are significant model terms. The “Lack of Fit F value” of 0.65 implies the Lack of Fit is not significant relative to the pure error. “Pred R 2” of 0.9187 is in reasonable agreement with the “Adj R 2” of 0.9705, i.e. the difference is <0.2.
Figure 3a–c shows the response surface plots for combined effect two variables (keeping the third constant) on percent Phosphate removal efficiency. Optimal conditions for phosphate removal were exactly located inside the design boundary as is obvious from the figure which shows an obvious trough in the response surfaces. Interactive effects between reaction time and pH, coagulant dose and pH and coagulant dose and reaction time were significant on phosphate removal. As can be seen from Fig. 3a, at a constant coagulant dose of 2.5 g/l, phosphate removal was highest at the central values and decreased with increase in reaction time and pH, and then again increased with further increase in reaction time and pH values. Plot of reaction time versus pH showed optimum region from pH range 9.3 to slightly above 12 and reaction timings from 49 to 150 min. Figure 3b, c, coagulant dose versus pH and coagulant dosage versus reaction time confirmed that the optimal conditions for phosphate removal were located in the region, where pH, coagulation dosage and reaction time ranged from 9.3 to above 12, 2.2–2.87 g/l and 60–154 min respectively.
Color removal
The Model F value of 83 implies the model is significant. A, B, C, AB, AC, BC, A2, B2, C2 are significant model terms. The “Lack of Fit F value” of 2.25 implies the Lack of Fit is not significant relative to the pure error. The “Pred R 2” of 0.9014 is in reasonable agreement with the “Adj R 2” of 0.9788, i.e. the difference is <0.2.
Figure 4a with change in reaction time and pH at constant coagulant dose of 2.5 g/l color removal shows a gradual but constant increase from 81 % (at pH 8 and time 30 min) to 86 % (pH 8 and 150 min) with increase in time, whereas, color removal exhibited gradual decrease with increase in pH and then became almost stable after pH 10 (76 % at pH 11.74 and time 30 min). As can be seen from Fig. 4b illustrating 3D plot of coagulant dose and pH at constant reaction time of 90 min, color removal showed a slight increase with increasing coagulant dose from 85 % (at pH 8 and conc. 2 g/l) to 86 % (pH 8 and concentration of coagulant = 3 g/l). However, from an initial removal of 85 % at pH 8, color removal decreased gradually to 79 % (at pH 12 and coagulant dosage of 2 g/l). The results were in accordance with some of the previous studies, e.g. Liu and co-workers reported an optimum pH of 8 and dose 10 g/l for 93.31 % reduction in color. Figure 4c plots the 3D interaction of coagulant dose and reaction time at constant reaction pH (10) on percent Color removal efficiency. After 30 min of reaction time, at pH 10 around 77 % of color was removed with FeCl3 and it increased with increase in FeCl3 dosage to approximately 80 % (with 3 g/l). With increasing retention time, color removal increased from 77 to 83 %. Researchers have conducted many studies using iron based coagulants for the treatment of landfill leachate. Zamora et al. (2000) reported a 50–70 % color reduction by a combined treatment employing activated carbon and coagulation. Aziz et al. (2007) investigated CF using four kinds of coagulant viz. alum, ferric(III) chloride, ferrous(II) sulphate and ferric (III) sulphate and recommended ferric chloride as a practicable coagulant to deal with decolorization of landfill leachate (achieved 94 % color removal at 800 mg/l of FeCl3 and pH 4). Jamali et al. (2009) reported 70 % color removal.
Sulfate removal
The Model F value of 380.19 implies the model is significant. A, C, AB, AC, BC, A2, B2, C2 are significant model terms. The “Lack of Fit F value” of 1.97 implies the Lack of Fit is not significant relative to the pure error. The “Pred R 2” of 0.9792 is in reasonable agreement with the “Adj R 2” of 0.953, i.e. the difference is <0.2.
Response surface plots for combined effect of reaction time and pH at constant coagulant dose of 2.5 g/l on sulfate removal from landfill leachate has been shown in Fig. 5a. As is obvious from the figure, sulfate removal showed a sharp increase with increase in pH as well as retention time. With increasing pH, it increased from 93 % (at 8) to 98.5 % (at 10.58 after 30 min of retention time) whereas, increase in retention time up to 150 min led to the removal of almost 99.5 % of sulfate from the Leachate sample. Sulfate removal gradually increased with increase in FeCl3 dose (Fig. 5b) from 97 % (at pH 8 and dose 2 g/l) to 99 % (at pH 8; 3 g/l). However, with increase in pH, removal efficiency exhibited an initial increase from 97 %, attained a maximum of 99 % (at pH 10) and then again decreased with any further increase in pH (97 % at pH 12). With increasing coagulant dose and retention time (Fig. 5c), sulfate removal efficiency improved slightly from 98 to 99 %. The results of the optimization study have been illustrated in Fig. 6a, b showing the contour plot numerical optimization and the overlay plot showing graphical optimization within the chosen criteria having the factors within range and maximized responses (COD, phosphate and color). Numerical optimization of the model revealed that the maximum reduction of 71, 93, 86 and 99.6 % COD, phosphate, color and sulfate could be achieved respectively at optimal FeCl3 dosage = 3 g/l, pH 8, and reaction time = 95 min; whereas the average EC (mS/cm), TDS (ppt) and salt (ppt) concentration in the effluent were 8.29, 6.35 and 4.35.
Conclusions
The objective of this study was to explore the optimum process conditions, using RSM, required while using FeCl3 as a coagulant to remove COD, phosphate and color from landfill leachate as well as to study the effect of FeCl3 addition on EC resulting from the treatment. Using BBD, on the basis of RSM, it was established that amalgamation of pH, coagulant dosage and reaction time has considerable effect on removal of the selected parameters. Maximum removal of COD was observed 71.3 % at pH 8.0, coagulant dose of 3 g/l and reaction time of 90 min. Maximum phosphate removal of 94 % was achieved at pH 8 after 30 min at a coagulant dosage of 2.5 g/l and after 90 min at a coagulant dosage of 2 g/l. Color removal of 86.1 was observed at pH 8 with 2.5 g/l of FeCl3 after 150 min of the reaction whereas 86 % of color was removed with 2 g/l of the coagulant after 90 min of the reaction. Almost complete removal (99.6 %) of sulfate was observed at pH 8 with 2.5 g/l of FeCl3 after 150 min of the reaction. As was obvious from “Prob > F” values <0.0500 model terms have important effect on removal using FeCl3 as a coagulant. FeCl3 can be significantly employed as a coagulant for pre-treatment or post-treatment of landfill leachate having high concentration of recalcitrant compounds rendering biological processes inefficient.
However, as is obvious from the study and earlier reports as well, the extent of removal may vary depending on the composition of the solid waste in the dumpsite and landfill leachate constituent compounds so that a preliminary study needs to be done so as to achieve higher removal efficiencies.
References
Abbas AA, Jingsong G, Ping LZ, Pan YY, Al-Rekabi WS (2009) Review on landfill leachate treatments. J Appl Sci Res 5:534–545
Abu Amr SS, Aziz HA, Bashir MJK (2014) Application of response surface methodology (RSM) for optimization of semi-aerobic landfill leachate treatment using ozone Appl. Water Sci. doi:10.1007/s13201-014-0156-z
Adlan MN, Palaniandy P, Aziz HA (2011) Optimization of coagulation and dissolved air flotation (DAF) treatment of semi-aerobic landfill leachate using response surface methodology (RSM). Desalination 277:74–82
Amokrane A, Comel C, Veron J (1997) Landfill leachate treatment pretreatment by coagulation–flocculation. Water Res 31:2775–2782
Anfruns A, Gabarró J, Gonzalez-Olmos R, Puig S, Balaguer MD, Colprim J (2013) Coupling anammox and advanced oxidation-based technologies for mature landfill leachate treatment. J Hazard Mater 258–259:27–34
Ayoub GM, Semerjian L, Acra A, El Fadel M, Koopman B (2001) Heavy metal removal by coagulation with seawater liquid bittern. J Environ Eng 127:196–202
Aziz HA, Alias S, Adlan MN, Faridah, Asaari AH, Zahari MS (2007) Colour removal from landfill leachate by coagulation and flocculation processes. Bioresour Technol 98:218–220
Bezerra MA, Santelli RE, Oliveiraa EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Boumechhour F, Rabah K, Lamine C, Said BM (2013) Treatment of landfill leachate using Fenton process and coagulation/flocculation. Water and Environ J 27:114–119
Castrillón L, Fernández-Nava Y, Ulmanu M, Anger I, Marañón E (2010) Physico-chemical and biological treatment of MSW landfill leachate. Waste Manag 30:228–235
de Torres-Socías E, Prieto-Rodríguez L, Zapata A, Fernández-Calderero I, Oller I, Malato S (2014) Detailed treatment line for a specific landfill leachate remediation: brief economic assessment. Chem Eng J. doi:10.1016/j.cej.2014.02.103
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box–Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186
Ghafari S, Aziz HA, Bashir MJK (2010) The use of poly-aluminum chloride and alum for the treatment of partially stabilized leachate: a comparative study. Desalination 257:110–116
Jadhav SB, Surwase SN, Phugare SS, Jadhav JP (2013) Response surface methodology mediated optimization of Remazol Orange decolorization in plain distilled water by Pseudomonas aeruginosa BCH. Int J Environ Sci Technol 10:181–190. doi:10.1007/s13762-012-0088-9
Jadhav SB, Chougule AS, Shah DP, Pereira CP, Jadhav JP (2014) Application of response surface methodology for the optimization of textile effluent biodecolorization and its toxicity perspectives using plant toxicity, plasmid nicking assays. Clean Tech Environ Policy. doi:10.1007/s10098-014-0827-3
Jamali HA, Mahvi AH, Nabizadeh N, Vaezi F, Omrani GA (2009) Combination of coagulation–flocculation and ozonation processes for treatment of partially stabilized landfill leachate of Tehran. World Appl Sci J 5(Special Issue for Environment):9–15
Jin Y, Wu Y, Cao J, Wu Y (2014) Optimizing decolorization of methylene blue and methyl orange dye by pulsed discharged plasma in water using response surface methodology. J Taiwan Inst Chem E 45–2:589–595
Kamaruddin MA, Yusoff MS, Aziz HA, Hung YT (2014) Sustainable treatment of landfill Leachate. Appl Water Sci. doi:10.1007/s13201-014-0177-7
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336
Kousha M, Daneshvar E, Sohrabi MS, Koutahzadeh N, Khataee AR (2012) Optimization of C.I. Acid black 1 biosorption by Cystoseira indica and Gracilaria persica biomasses from aqueous solutions. Int Biodeterior Biodegrad 67:56–63
Kurniawan TA, Lo WH (2009) Removal of refractory compounds from stabilized landfill leachate using an integrated H2O2 oxidation and granular activated carbon (GAC) adsorption treatment. Water Res 43:4079–4091
Kurniawan TA, Lo W, Chan GYS (2006) Radicals-catalyzed oxidation reactions for degradation of recalcitrant compounds from landfill leachate. Chem Eng J 125:35–57
Liu X, Li XM, Yang Q, Yue X, Shen TT, Zheng W, Luo K, Sun YH, Zeng GM (2012) Landfill leachate pretreatment by coagulation–flocculation process using iron-based coagulants: Optimization by response surface methodology. Chem Eng J 200–202:39–51
Moradi M and Ghanbari F (2014) Application of response surface method for coagulation process in leachate treatment as pretreatment for Fenton process: Biodegradability improvement. J Water Proc Eng. 4:67–73
Mor S, Khaiwal R, Dahiya RP, Chandra A (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess 118:435–456. doi:10.1007/s10661-006-1505-7
Nair AT, Ahammed MM (2013) The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J Clean Prod. doi:10.1016/j.jclepro.2013.12.037
Nordin MY, Venkatesh VC, Sharif S, Elting S, Abdullah A (2004) Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 104 steel. J Mater Process Technol 145:46–58
Oller I, Malato S, Sánchez-Pérez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination: a review. Sci Total Environ 409:4141–4166
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150:468–493
Rivas FJ, Beltran F, Carvalho F, Acedo B, Gimeno O (2004) Stabilized leachate: sequential coagulation–flocculation + chemical oxidation process. J Hazard Mater B116:95–102
Sari H, Yetilmezsoy K, Ilhan F, Yazici S, Kurt U, Apaydin O (2013) Fuzzy-logic modeling of Fenton’s strong chemical oxidation process treating three types of landfill leachates. Environ Sci Pollut Res 20:4235–4253
US Environmental Protection Agency (EPA) (2002) Municipal solid waste in the United States: 2000 facts and figures, Office of Solid Waste and Emergency Response, Washington DC (EPA 530-R-02-001)
Wang JP, Chen YZ, Ge XW, Yu HQ (2007) Optimization of coagulation–flocculation process for a paper-recycling wastewater treatment using response surface methodology. Colloid Surf A 302:204–210
Wang X, Han J, Chen Z, Jian L, Gu X, Lin CJ (2012) Combined processes of two-stage Fenton-biological anaerobic filter-biological aerated filter for advanced treatment of landfill leachate. Waste Manag 32:2401–2405
Wang P, Zeng G, Peng Y, Liu F, Zhang C, Huang B, Zhong Y, He Y, Lai Y (2014a) 2,4,6-Trichlorophenol-promoted catalytic wet oxidation of humic substances and stabilized landfill leachate. Chem Eng J 247:216–222
Wang Y, Chen K, Mo L, Li J, Xu J (2014b) Optimization of coagulation–flocculation process for papermaking-reconstituted tobacco slice wastewater treatment using response surface methodology. J Ind Eng Chem 20:391–396
Wu X, Zhang H, Li Y, Zhang D, Li X (2014) Factorial design analysis for COD removal from landfill leachate by photoassisted Fered-Fenton process. Environ Sci Pollut Res 21:8595–8602
Xiao S, Peng J, Song Y, Zhang D, Liu R, Zeng P (2013) Degradation of biologically treated landfill leachate by using electrochemical process combined with UV irradiation. Sep Purif Technol 117:24–29
Zamora RM, Moreno A, Orta de Velasquez M, Ramirez I (2000) Treatment of landfill leachates by comparing advanced oxidation and coagulation–flocculation processes coupled with activated carbon adsorption. Water Sci Technol 41:231–235
Zhao R, Gupta A, Novak JT, Goldsmith CD, Driskill N (2013a) Characterization and treatment of organic constituents in landfill leachates that influence the UV disinfection in the publicly owned treatment works (POTWs). J Hazard Mater 258–259:1–9
Zhao X, Wei X, Xia P, Liu H, Qu J (2013b) Removal and transformation characterization of refractory components from biologically treated landfill leachate by Fe2+/NaClO and Fenton oxidation. Sep Purif Technol 116:107–113
Acknowledgments
I would like to express my sincere thanks to University Grant Commission, New Delhi for awarding Basic Scientific Research (BSR) fellowship in the form of Senior Research Fellowship (SRF) for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kumar, S.S., Bishnoi, N.R. Coagulation of landfill leachate by FeCl3: process optimization using Box–Behnken design (RSM). Appl Water Sci 7, 1943–1953 (2017). https://doi.org/10.1007/s13201-015-0372-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0372-1