Abstract
The present study provides information about the binding of Pb(II) ions on an eco-friendly and easily available biodegradable biomass Trifolium resupinatum. The powdered biomass was characterized by FTIR, potentiometric titration and surface area analyses. The FTIR spectrum showed the presence of hydroxyl, carbonyl and amino functional groups and Pb(II) ions bound with the oxygen- and nitrogen-containing sites (hydroxyl and amino groups). The acidic groups were also confirmed by titrations. Effects of various environmental parameters (time, pH and concentration) have been studied. The biosorption process achieved equilibrium in a very short period of time (25 min). Non-linear approach for Langmuir and Freundlich models was used to study equilibrium process and root mean-square error was used as an indicator to decide the fitness of the mathematical model. The biosorption process was found to follow pseudo-second-order kinetics and was very fast. Thus, the biomass can be cost-effectively used for the binding of Pb(II) ions from aqueous solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of heavy metal ions are essential in diverse physiological functions and thus are essential for life. However, heavy metals become toxic to human beings when they exceed certain allowed levels (Reddy et al. 2010b). Contamination of aquatic systems due to toxic heavy metal ions has become a problem of global concern (Yan et al. 2010). These pollution-causing heavy metals come from various industrial sources such as mining, photographic industries, battery manufacture, paints manufacture, electroplating, cosmetics, etc. Natural waters also contain toxic metals depending on bedrocks (Obuseng et al. 2012).
Lead and lead compounds are generally toxic pollutants (EPA safe limit is 0.015 mg/L (Obuseng et al. 2012). Due to acute toxicity, lead along with mercury (Hg) and cadmium (Cd) forms “the big three” toxic metals with great hazards (Akar et al. 2012). Lead ions cause a number of disorders such as behavior and learning disabilities, vomiting, slow growth, neurotoxin (Riaz et al. 2009) and problems of the gastrointestinal and reproductive tract (Ibrahim et al. 2012). Lead compounds are known to be metal poison and enzyme inhibitor (Munagapati et al. 2010).
Conventional treatment methods for lead-contaminated waters are costly and produce sludge which requires further treatments. The modern and developing technique for metal removal is biosorption—a process of heavy metal removal by ‘dead’ biological materials (Bingöl et al. 2012). Biosorption by materials from higher plants is a relatively low-cost process. Several workers have investigated and reported the ability of materials from various plants. Peels from Punica granatum (Ay et al. 2012), Moringa oleifera seeds biomass (Obuseng et al. 2012), M. oleifera leaves (Reddy et al. 2010a), M. oleifera bark (Reddy et al. 2010b), Gossypium hirsutum (cotton) waste (Riaz et al. 2009), Pinus sylvestris cone biomass (Ucun et al. 2003), Calophyllum inophyllum seed husk (Lawal et al. 2010), Agave sisalana (sisal fiber) (dos Santos et al. 2011) and straw from Triticum aestivum (Farooq et al. 2007) are among the explored materials for the adsorption of different metals from their aqueous solutions.
To the best of our knowledge, no study has been reported for the potential use of material from Trifolium resupinatum regarding biosorption. The aim of the present study is to explore the binding of Pb(II) ions onto this biomass and study the effect of various parameters on it. The interaction of Pb(II) ions with various functional groups has also been reported. There are different factors which made it a good biosorbent. It is easily available. The rate of growth is fast and a new crop can be obtained after 15–20 days. Since its crop can be harvested seven to nine times and is available for 5–6 months, the biosorptive removal of Pb(II) ions using T. resupinatum can be performed on a large scale easily.
Materials and methods
All the chemicals used in the present study were directly obtained from Merck (Germany). Distilled water was used for all kinds of solution preparation and dilutions. The stock solution of Pb(II) ions (1,000 mg/L) was prepared by dissolving a known amount of lead(II) nitrate in water.
Collection and preparation of biomass
The plants of T. resupinatum were collected from farmlands of the University of Punjab, Lahore (31.492414°, 74.307382°). These plants were first washed with tap water to remove any dust/dirt particles and then with distilled water. The washed plants were dried first under shade in a laboratory and then in an electric oven (Binder, Germany) at 105 °C to constant mass. The dried plants were first manually crushed and then ground to powder (blender/dry mill, MJ 2001). The powdered biomass, designated as TRB, was stored in airtight bottle for further use.
Characterization of the biomass
The presence of functional groups in TRB was identified by FTIR analysis using standard KBr disc method. The surface area of TRB was determined by using methylene blue as an adsorbent. The method is discussed elsewhere (Abia and Asuquo 2007). The concentration of strong and weak acidic groups present on TRB was determined by potentiometric and conductometric titrations (Murphy et al. 2007).
Batch biosorption experiments
The batch biosorption studies were carried out in a series of conical flasks (250 mL). The effect of time of contact (5–45 min) was studied using a known amount of TRB in a solution of Pb(II) ions (50 mL, 50 mg/L) at predefined pH and at a specific temperature. The flasks were shaken on an orbital shaker (Vortex OSM-747) at an agitation speed of 150 rpm. After a regular time interval, the suspensions were filtered and the filtrate was analyzed using an atomic absorption spectrophotometer (Perkin-Elmer AAnalyst 100) to determine the equilibrium concentration of Pb(II) ions.
The amount of Pb(II) ions sorbed at equilibrium per unit mass of the adsorbent material (qe, mg/g) was calculated as follows:
where C0 and C e are the initial and equilibrium concentrations of Pb(II) ions, respectively. V is the volume of the solution in mL and m is the mass of the dry biomass in mg. All the experiments were performed in triplicate and the results were reported as the mean values. OriginPro® was used for plotting graphs. Regression analysis (R2 value) and the comparison of experimental and calculated values from the mathematical models by root mean-square errors (RMSE) were performed to discuss the fitting of the models. Blank experiments were performed to study the adsorption of metal ions by glassware. No detectable adsorption by glassware was found.
Results and discussion
Characterization of TRB
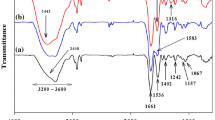
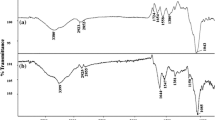
Fourier transformed infrared (FTIR) spectra were obtained for simple and Pb(II)-loaded TRB (Fig. 1). The spectrum for simple TRB revealed various functional groups in TRB which are responsible for biosorption. These groups include hydroxyl (–OH) group and amino group (–NH2) having peak at 3292.84 cm−1, alkyl (–R, CHn) group having peak at 2926.01 cm−1, C=O bond of carboxyl group and their esters having a peak at 1597.06 cm−1, and other peaks at 1421.54, 1346.31 and 1299.87 cm−1 for asymmetric stretching of the carboxylate ion (C–O) or phosphate double bond. Thus, biomass is rich in the oxygen-containing functional groups.
When the FTIR spectrum of TRB was compared with that of Pb(II)-loaded TRB, a marked difference was recorded. A significant shift was observed in the broad band of hydroxyl and/or amino groups from 3292.84 to 3414.00 cm−1. The strong shift indicated a strong interaction between oxygen of the hydroxyl (–OH) group of carboxylic acid and/or nitrogen of the amino group (–NH2) and Pb(II) ions. Similarly, a change at 1597.06–1643.35 for C=O bond of carboxyl group was also observed indicating a strong interaction between carbonyl oxygen and Pb(II) ions. This led to conclude that O- and/or N-containing groups are the main sites for biosorption of Pb(II) ions on TRB.
The comparison of FTIR spectra of simple and metal-loaded TRB revealed a strong interaction between acidic groups (carboxylic acid/carbonyl) and the Pb(II) ions. So potentiometric titrations with standard sodium hydroxide were performed to determine the concentration of acidic groups in TRB (figure not shown). The acidic groups present on the TRB surface and their corresponding pKa values were determined by categorizing the inflection points in the curve. Reading the location of each peak on the x-axis (NaOH, mmol/g) gave the concentration of the acidic groups of TRB. The first peak (corresponding to 0.8 mmol/g of NaOH) of the plot gave the number of strong acidic groups, whereas the final peak gave the number of total acidic groups (corresponding to 8.2 mmol/g of NaOH). The number of weak acidic groups was calculated from the difference of strong and weak acidic groups. Once these values were established, the corresponding pKa values were then identified from the original titration curve (Murphy et al. 2007).
The pKa values and the number of acidic groups on the TRB surface are given in Table 1. It is indicated that the surface of TRB contains a great number of acidic functionalities. FTIR analysis shows that carboxyl groups (relatively weak acids), on the surface of TRB, are primarily responsible for biosorption of Pb(II) ions in suspension. It was expected that the TRB species would exhibit superior biosorption performance to a number of plant biomasses. Hydroxyl groups in polysaccharides of cell wall of TRB are considerably weaker than carboxyl groups and therefore may only interact with cations at a higher pH. This usually occurs at pH > 10. Therefore, surface hydroxyl groups play a significant role in binding at very high pH values. Proteins have also been known to interact with metal ions, particularly between pH 6–9 and protonated amino groups have a pKa value of around 8. TRB displayed at least one pKa value, i.e., 10.7 in this region (Murphy et al. 2007).
The specific surface area of simple as well as metal-loaded TRB was determined using the methylene blue absorption (MBT) method described by (Abia and Asuquo 2007). The specific surface area of sorbed methylene blue was calculated using the formula
where m AB is the amount of methylene blue sorbed on the surface of biomass after complete cation replacement, M S the mass of biomass, A V the Avogadro’s number (6.02 × 1023) and A MB the area covered by one methylene blue molecule (typically assumed to be 130 Å2) (Hang and Brindley 1970). The active surface area of TRB was calculated to be 18.33 m2/g and the surface area of Pb(II) ions loaded was 20.08 m2/g. This increase in surface area may be due to the chelating effect of Pb(II) ions toward methylene blue molecules. As more molecules of methylene blue were attracted by Pb(II) ions, this resulted in increase in the surface area of metal-loaded TRB.
Biosorption kinetics
The contact time studies provide information about the minimum time required to bind the maximum amount of Pb(II) ions at the liquid–solid interface and thus help in scaling up the process. The optimum (equilibrium) time helps in studying the rate of the binding process.
The effect of the time of contact on the binding of Pb(II) by TRB is shown in Fig. 2. It can be observed that the biosorption of Pb(II) ions on TRB was rapid during the first 20 min. The curve became parallel to the time axis after 25 min. The binding of Pb(II) ions on TRB reached equilibrium at 25 min. So a time of 25 min was considered as equilibrium time of contact for Pb(II) ions.
For biosorption of Pb(II) ions, a number of studies have been reported showing different equilibrium times of contact. A time of 4 h (240 min) to 45 min has been reported for the maximum binding/adsorption of Pb(II) on various biosorbents originating from various higher plants (Gundogd et al. 2009; Munagapati et al. 2010; Ucun et al. 2003; García-Rosales and Colín-Cruz 2010; Bingöl et al. 2012; Ay et al. 2012; Xiangliang et al. 2005) The biosorption of Pb(II) ions by T. resupinatum (present study) shows quite less equilibrium time (25 min). This indicates the advantage of the biomass in the present study over a number of already reported ones.
The kinetic characteristics of the sorbent depend not only on the presence of the active binding sites, but also on the accessibility of the Pb(II) ions without steric hindrance. This means that the mechanism of biosorption depends on the physical and chemical characteristics of the sorbent as well as on the mass transfer process. The kinetics of Pb(II) ions sorption onto TRB was studied by famous kinetic models, i.e., pseudo-first-order and pseudo-second-order (PSO) models. The linear forms of both the models are, respectively, shown as
where q e and q t (mg/g) are the amounts of metal sorbed at equilibrium and at the given the time t, respectively, and k1 (1/min) and k2 (mg/g min) are the first- and second-order rate constants, respectively.
Pseudo-first-order model is based on the fact that the rate of biosorption is proportional to the number of free sites present on the biomass. If the pseudo-first-order kinetics is applicable to equilibrium data, a plot of ln(q e − q t ) versus t should provide a straight line. The value of k1 can be determined from slope and predicted q e can be determined from the intercept of the plot. The variation in rate should be proportional to the first power of concentration for strict surface adsorption. However, the relationship between initial solute concentration and rate of adsorption will not be linear when pore diffusion limits the adsorption process. The values of kinetic parameters are given in Table 2. The value of k1 was 0.104 min−1. The R2 value (0.979) shows that pseudo-first-order can describe the Pb(II) ions biosorption by TRB. However, when q e was calculated using the model value and compared with the experimental value, it was found that the experimental value of q e (2.83 mg/g) was not in agreement with the calculated value of q e (0.4445 mg/g). It can be inferred that the biosorption of Pb(II) ions onto TRB did not follow pseudo-first-order kinetics. From literature survey, it was observed that most of the divalent ions do not follow pseudo-first-order kinetics (Table 3).
The PSO model is based on the assumption that biosorption follows a second-order mechanism, whereby the rate of sorption is proportional to the square of the number of unoccupied sites. The second-order plot of t/q t versus t from the above equation resulted in a straight line for the biosorption of Pb(II) ions onto TRB and led to the determination of the PSO rate constants k2 and q e from the slope and the intercept (Table 2). The calculated q e value (2.89 mg/g) was very close to the experimentally determined one (2.83 mg/g). The R2 value for the PSO kinetic model was 0.999 for the biosorption Pb(II) ions onto TRB. The value of k2 was 0.5030 g/mg/min.
On the basis of theoretical consideration, the biosorption of divalent metal ions (M) onto two free binding sites (B) can be explained by the following expressions:
It means that the biosorption rate would be proportional to the concentration of metal ions and the square of the number of free sites onto TRB, which corresponds to the term (q e − q t )2 in the PSO model. The best fit of the PSO model indicates that a 1:2 binding stoichiometry applies, where one divalent metal binds to two monovalent binding sites (Lasheen et al. 2012). This is in accordance to a number of already reported results in literature, which show that biosorption of divalent materials mostly followed the PSO kinetic model (Table 3).
Equilibrium modeling: effect of Pb(II) concentration and adsorption isotherms
Equilibrium modeling or adsorption isotherms are studied to characterize the adsorption process. These represent the relationship between the amounts of substance adsorbed on the adsorbent and amount of adsorbate remained in the solution. Two of the most commonly used equilibrium models, i.e., Langmuir and Freundlich models have been employed to study the equilibrium process in the present study.
The Langmuir model represents maximum adsorption capacity. It is based on the principle that the Pb(II) ions are adsorbed on the outer heterogeneous surface of the adsorbent forming a monolayer coverage. The Freundlich model estimates the adsorption intensity of adsorbate on adsorbent. It is based on multilayer adsorption on heterogeneous surface. Both the models are shown as follows
where q m is the maximum uptake capacity (mg/g), KL (L/mg) the Langmuir adsorption constant, KF the Freundlich constant related to adsorption capacity (mg/g) and C e the concentration of metal ions at equilibrium (mg/L).
The non-linear plot for both the models is shown in Fig. 3. The Langmuir and Freundlich constants are given in Table 4. The experimental data were quite in agreement with the Langmuir model and thus indicated a monolayer coverage of the surface of TRB with the metal ions. Similar inference can be deduced from the R2 value. The maximum biosorption capacity of TRB for Pb(II) ions was found to be 10.38 mg/g. The feasibility of the Langmuir model was also monitored by using a dimensionless parameter RL as follows:
The value of RL indicates the type of curve as irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1) and unfavorable (RL > 1) (Farooq et al. 2010b). The calculated RL values for the binding of Pb(II) ions by TRB were between 0.8916 and 0.2842. Hence, the binding of Pb(II) ions was feasible under the studied conditions.
Langmuir biosorption capacity was also used to calculate the specific surface area (SL, m2/g) of the monolayer biosorption of Pb(II) by TRB as follows
where N is the Avogadro’s number (6.022 × 1023), A the cross-sectional area of metal ion (Å2) and M the atomic mass of the metal ion. The cross-sectional area of Pb(II) is 5.56 Å2 (Ho et al. 2002). The surface area thus calculated was 1.6789 m2/g.
The Freundlich model was also used to study the equilibrium of Pb(II) binding by TRB (Fig. 3). R2 value was 0.9652 which is close to 0.98. It can be observed that the curve for the ‘model’ was not in agreement with the experimental data points. This indicated that the Freundlich model was not suitable to discuss the equilibrium of the process under study.
The agreement between the experimental and ‘model’ values was analyzed using RMSE. The greater the value of RMSE, the greater is the experimental data in disagreement with the model or predicted values. It can be seen that the RMSE value was smaller for the Langmuir model as compared to the Freundlich model. This is in agreement with the above argument that the Langmuir model describes the equilibrium of the binding of Pb(II) ions by TRB.
Effect of pH
The pH of an aqueous solution is probably the most important parameter for effective biosorption of metal ions on to the biomass. It is directly related to the competitive ability of hydrogen ions and metal ions to bind to active sites present on the biomass. Generally, metal biosorption involves complex mechanisms of ion exchange, chelation, adsorption by physical forces and ion entrapment in inter- and intrafibrillar capillaries and spaces of the cell structural network of a biosorbent (Munagapati et al. 2010). FTIR spectroscopic analysis showed that TRB had a variety of functional groups, such as carboxyl, hydroxyl and amine, involved in almost all potential binding mechanisms (Farooq et al. 2010a). Moreover, depending on the pH values of the aqueous solutions, these functional groups participate in metal ion bindings.
The effect of pH on the biosorption of Pb(II) ions onto TRB was studied at pH 1–9 and the results are given in Fig. 4. The maximum biosorption was observed at pH 3.0 after which q e became parallel to the pH axis. At very low pH, the concentration of H+ ions was high. This led to the development of positive charge on the active sites of biomass and also a competition between Pb(II) ions and H+ in the bulk of solution to attach with the active binding sites of TRB. So, there was a minimum binding of Pb(II) ions at low pH. As the pH of a solution increases, TRB becomes less positive and the concentration of H+ ions also decreases. Thus, there is less competition of Pb(II) ions with H+ ions and this resulted in more biosorption. So, it was concluded that optimum pH for biosorption of Pb(II) ion on TRB was 3.0. The biosorption of Pb(II) ions at low pH follows various other mechanisms in addition to the simple ion exchange.
Conclusion
The present study is aimed at exploring the biomass from T. resupinatum for the removal of toxic metal Pb(II) ions from aqueous solution. The effect of various environmental parameters such as time, pH and concentration on the binding of Pb(II) ions by T. resupinatum has been studied. It was found that the optimum time of contact was 25 min at an optimum pH of 3 (fast process). Increasing the dose of biomass increased the binding capacity of the biomass. The equilibrium of binding of Pb(II) ions on to biomass was studied by Langmuir and Freundlich models. A non-linear approach was used to study the fitting of the mathematical model to the experimental data. RMSE was used to compare the agreement between the mathematical model and the experimental data. It was found that the biosorption process followed the Langmuir model with q m of 10.38 mg/g, thus forming a monolayer of Pb(II) ions on the surface of the biomass. The kinetics of the process was studied by pseudo-first-order and PSO models. The process was found to follow the PSO kinetics. The results indicated that the non-toxic biodegradable biomass from T. resupinatum can be used for the cost-effective removal of Pb(II) ions from aqueous solution.
References
Abia AA, Asuquo ED (2007) Kinetics of Cd2+ and Cr3+ sorption from aqueous solutions using mercaptoacetic acid modified and unmodified oil palm fruit fibre (Elaeis guineensis) adsorbents. Tsinghua Sci Technol 12:485–492
Akar ST, Gorgulu A, Anilan B, Kaynak Z, Akar T (2009) Investigation of the biosorption characteristics of lead(II) ions onto Symphoricarpus albus: batch and dynamic flow studies. J Hazard Mater 165:126–133
Akar ST, Arslan S, Alp T, Arslan D, Akar T (2012) Biosorption potential of the waste biomaterial obtained from Cucumis melo for the removal of Pb2+ ions from aqueous media: equilibrium, kinetic, thermodynamic and mechanism analysis. Chem Eng J 185–186:82–90
Ay CÖ, Özcan AS, Erdoğan Y, Özcan A (2012) Characterization of Punica granatum L. peels and quantitatively determination of its biosorption behavior towards lead(II) ions and Acid Blue 40. Coll Surf B: Biointerfaces 100:197–204
Bingöl D, Hercan M, Elevli S, Kılıç E (2012) Comparison of the results of response surface methodology and artificial neural network for the biosorption of lead using black cumin. Bioresour Technol 112:111–115
Blázquez G, Calero M, Hernáinz F, Tenorio G, Martín-Lara MA (2010) Equilibrium biosorption of lead(II) from aqueous solutions by solid waste from olive-oil production. Chem Eng J 160:615–622
Cruz-Olivares J, Pérez-Alonso C, Barrera-Díaz C, Natividad R, Chaparro-Mercado MC (2011) Thermodynamical and analytical evidence of lead ions chemisorption onto Pimenta dioica. Chem Eng J 166:814–821
dos Santos WNL, Cavalcante DD, da Silva EGP, das Virgens CF, Dias FS (2011) Biosorption of Pb(II) and Cd(II) ions by Agave sisalana (sisal fiber). Microchem J 97:269–273
Farooq U, Khan MA, Athar M (2007) Triticum aestivum: a novel biosorbent for lead (II) ions. Agrochimica 51:309–318
Farooq U, Kozinski JA, Khan MA, Athar M (2010a) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101:5043–5053
Farooq U, Kozinski JA, Khan MA, Athar M (2010b) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101:5043–5053
García-Rosales G, Colín-Cruz A (2010) Biosorption of lead by maize (Zea mays) stalk sponge. J Environ Manag 91:2079–2086
Gundogd A, Ozdes D, Duran C, Bulut VN, Soylak M, Senturk HB (2009) Biosorption of Pb(II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem Eng J 153:62–69
Han R, Zhang J, Zou W, Shi J, Liu H (2005) Equilibrium biosorption isotherm for lead ion on chaff. J Hazard Mater B125:266–271
Hang PT, Brindley GW (1970) Methylene blue absorption by clay minerals. Determination of surface areas and cation exchange capacities (Clay-organic studies XVIII). Clays Clay Miner 18:203–212
Ho YS, Huang CT, Huang HW (2002) Equilibrium sorption isotherm for metal ions of tree fern. Process Biochem 37:1421–1430
Ibrahim HS, Ammar NS, Soylak M, Ibrahim M (2012) Removal of Cd(II) and Pb(II) from aqueous solution using dried water hyacinth as a biosorbent. Spectrochim Acta Part A Mol Biomol Spectrosc 96:413–420
Lasheen MR, Ammar NS, Ibrahim HS (2012) Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: equilibrium and kinetic studies. Solid State Sci 14:202–210
Lawal OS, Sanni AR, Ajayi IA, Rabiu OO (2010) Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead(II) ions onto the seed husk of Calophyllum inophyllum. J Hazard Mater 177:829–835
Munagapati VS, Yarramuthi V, Nadavala SK, Alla SR, Abburi K (2010) Biosorption of Cu(II), Cd(II) and Pb(II) by Acacia leucocephala bark powder: kinetics, equilibrium and thermodynamics. Chem Eng J 157:357–365
Murphy V, Hughes H, McLoughlin P (2007) Cu(II) binding by dried biomass of red, green, and brown macroalgae. Water Res 41:731–740
Obuseng V, Nareetsile F, Kwaambwa HM (2012) A study of the removal of heavy metals from aqueous solutions by Moringa oleifera seeds and amine-based ligand 1,4-bis[N, N-bis(2-picoyl)amino]butane. Anal Chim Acta 730:87–92
Ramana DKV, Reddy DHK, Yu JS, Seshaiah K (2012) Pigeon peas hulls waste as potential adsorbent for removal of Pb(II) and Ni(II) from water. Chem Eng J 197:24–33
Reddy DHK, Harinath Y, Seshaiah K, Reddy AVR (2010a) Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162:626–634
Reddy DHK, Seshaiah K, Reddy AVR, Rao MM, Wang MC (2010b) Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: equilibrium and kinetic studies. J Hazard Mater 174:831–838
Riaz M, Nadeem R, Hanif MA, Ansari TM, Rehman KU (2009) Pb(II) biosorption from hazardous aqueous streams using Gossypium hirsutum (Cotton) waste biomass. J Hazard Mater 161:88–94
Ucun H, Bayhan YK, Kaya Y, Cakici A, Algurb OF (2003) Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 154:233–238
Waseem S, Din MI, Nasir S, Rasool A (2012) Evaluation of Acacia nilotica as a non conventional low cost biosorbent for the elimination of Pb(II) and Cd(II) ions from aqueous solutions. Arab J Chem. http://dx.doi.org/10.1016/j.arabjc.2012.03.020
Xiangliang P, Jianlong W, Daoyong Z (2005) Biosorption of Pb(II) by Pleurotus ostreatus immobilized in calcium alginate gel. Process Biochem 40:2799–2803
Yan C, Li G, Xue P, Wei Q, Li Q (2010) Competitive effect of Cu(II) and Zn(II) on the biosorption of lead(II) by Myriophyllum spicatum. J Hazard Mater 179:721–728
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Athar, M., Farooq, U., Aslam, M. et al. Adsorption of Pb(II) ions onto biomass from Trifolium resupinatum: equilibrium and kinetic studies. Appl Water Sci 3, 665–672 (2013). https://doi.org/10.1007/s13201-013-0115-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0115-0