Abstract
Environmental contamination due to anthropogenic and natural sources is increasing day by day because of increase in population, industrialization and urbanization. Arsenic species released into the environment tend to persist indefinitely, circulating and eventually accumulating throughout the food chain, thus becoming a serious threat to the environment. The present study explores the effectiveness of Leucaena leucocephala seed powder (agricultural waste) in removing arsenic ions from aqueous solution. Batch studies were carried out to characterize As (III) and As (V) removal capability of L. leucocephala seed powder. Maximum biosorption capacity for As (III) and As (V) was found to be 81.88 and 92.61 %, respectively. Amino acid–arsenic interaction has been highlighted on the basis of shifting of FTIR bands of native LLSP. Morphological changes and reduction in pore area have been observed in modified LLSP. Modification on the native LLSP results into the increase in percentage sorption of As (III) and As (V) up to 85 and 99 %, respectively. Enhancement in the percentage sorption is due the increase in the stability of the biosorbent due to increase in the final decomposition temperature of the modified LLSP. The findings showed that L. leucocephala seed powder can easily be envisaged as a new, vibrant, low-cost biosorbent for arsenic clean-up operations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (metalloid) is one of the most important pollutants and is naturally found in the environment. It is the only known human carcinogen for which there is adequate evidence of carcinogenic risk by both inhalation and ingestion. Arsenic poisoning has become a major threat to human society owing to the ever-increasing contamination of water. The source of arsenic pollution is from the discharge of various industries such as smelting, petroleum- refining, and pesticide, herbicide, glass and ceramic manufacturing industries (Gonzaga et al. 2006). The presence of arsenic in the environment, its toxicity and health hazards are well known and have been reviewed extensively (Kundu and Gupta 2006; Choonga et al. 2007). Arsenic toxicity causes skin lesions, damage mucous membranes, nervous system, gastrointestinal, cardiovascular, genotoxic, mutagenic and carcinogenic effects (Rivas and Aguirre 2010; Bose et al. 2011). Because of its high toxic effects, the World Health Organization (WHO) has revised the guidelines for arsenic in drinking water from 50 to 10 μg/L (Mohan et al. 2007). However, arsenic concentrations, about 100 times more than permissible limit, are found in many parts of world (Mandal and Suzuki 2002; Pereira et al. 2007). Contaminated drinking water has been found in Argentina, Chile, Mexico, China, India, Hungary, Bangladesh and Vietnam. Of these regions, India and Bangladesh are most seriously affected in terms of the size of the population at risk and the magnitude of the health problems (Wang and Wai 2004; Sohel et al. 2009). Approximately 30–35 million people in Bangladesh and 7 million people in West Bengal (India) are exposed to elevated levels of arsenic in drinking water. Arsenic concentrations were found from >0.5 to 3,200 μg/L, with As (III) present as the dominant species.
Removal of arsenic from contaminated water to satisfy drinking water standards has been a challenge for water authorities. The chemistry of arsenic is complex and interesting. In general, more toxic As (III), which exists predominantly as nonionic H3AsO3 in natural waters, is more difficult to remove compared with As (V), which exists as deprotonated oxy anions H2AsO4− or HAsO −24 (Burriel et al. 2006). Conventional methods for metal removal include chemical precipitation, lime coagulation, ion-exchange, reverse osmosis and flocculation (with various synthetic coagulants such as aluminium, ferric salts, soda ash, polymers, etc.) have been developed for arsenic removal (Nemade et al. 2007; Shao et al. 2008; Ranjan et al. 2009). These conventional methods for the removal of arsenic are associated with several disadvantages like unpredictable arsenic ion removal, high material costs and the generation of toxic sludge the disposal of which is a burden on the techno-economic feasibility of treatment procedures.
The search for new technologies involving the removal of arsenic from water bodies has directed attention to biosorption, based on arsenic binding capacities of agricultural wastes. Bioremediation involves processes that reduce overall treatment cost through the application of agricultural residues which are particularly attractive as they lessen reliance on imported water treatment chemicals, negligible transportation requirements and offer genuine, localized and appropriate solutions to water quality problems (Raj et al. 2010; Kardam et al. 2010). Biological materials such as algae, bacteria, fungi, yeast and agricultural waste products like water hyacinth, sunflower stalk, coconut coir pith, rice husk and grape stalk waste have been recognized as cheap natural sorbents for the removal of toxic metals (Anirudhan and Unnithan 2007; Alvarado et al. 2008; Malik et al. 2009; Yeneneh et al. 2011). Current research is oriented towards the enhancement in the sorption capacity and reusability of the biosorbent making it suitable for commercialization.
Leucaena leucocephala (subabul) is a tropical wild plant which does not need man-made irrigation and abundantly available throughout the year. The seeds of this plant are produced in large amounts and considered as an agricultural waste (Patil and Shrivastava 2010). The present piece of work reports the enhancement in the sorption potential of L. leucocephala seed powder (LLSP) for arsenite and arsenate from water bodies.
Materials and methods
Biosorbent preparation
Leucaena leucocephala tree was notified in the nearby area of Dayalbagh Educational Institute, Agra and the seeds were collected from the target plant. Seeds were washed thoroughly with double distilled water to remove the adhering dirt, dried at 65 °C for 24 h, crushed and sieved through (105 μm) mesh copper sieve.
Biosorption studies
Sorption studies using standard practices were carried out in batch experiments (triplicates) as a function of biosorbent dosage (2.0–6.0 g), contact time (10–60 min), arsenic concentration (0.05–50 μg/mL), volume of the solution 200 mL and pH (2–10). The solutions of sodium arsenite and sodium arsenate were taken in an Erlenmeyer flask. After pH adjustments, a known quantity of dried biosorbent was added and the arsenic-bearing suspensions were kept under magnetic stirring until the equilibrium conditions were reached. After shaking, the suspensions were allowed to settle down. The residual biosorbent sorbed with the arsenic ions were filtered using a Whatman 42 filter paper (Whatman International Ltd., Maidstone, England). The filtrates were collected and subjected to arsenic ion estimation using Hydride Generation Atomic Absorption Spectroscopy (Perkin Elmer 2380). The arsenic concentrations before and after adsorption were recorded. The per cent metal sorption by the sorbent was computed using the following equation:
where Co and Ce are the initial and final concentration of arsenic ions in the solution, respectively.
Modification on LLSP
Dried LLSP (10 g) was placed in pyridine (2.5 mL) in chloroform (95 mL), followed by drop wise addition of 4-bromobutyryl chloride (5 mL). The reaction mixture was sealed and gently stirred at 25 °C for 12 h. The acylated biosorbent was rinsed with chloroform to remove any unreacted 4-bromobutyryl chloride before being immersed in a mixture containing 10 g polyethylenimine (PEI) and KOH (0.1 g) in tert-amyl alcohol (90 mL). After the mixture was stirred at 75 °C for 24 h, the modified LLSP was rinsed with copious quantities of methanol and deionized water and then freeze-dried to constant weight.
Evidences in support of modified LLSP
To access the role of functional groups present on the surface of the biosorbent that might be involved in arsenic sorption, FTIR analysis in solid phase was performed using a Shimadzu 8400 Fourier Transform Infrared spectroscopy. Spectra of the native and modified LLSP were recorded. The morphological characteristics of LLSP were evaluated using Steroscan 360, Scanning Electron Microscope (Cambridge, UK). The Scanning Electron Micrographs of native and modified biosorbent at bar length equivalent to 10 μm, working voltage 20 kV with 700× magnification were recorded. TGA was performed using Thermo Gravimetric Analyzer (DTG-60, Shimadzu). The comparison of Initial Decomposition Temperature (IDT) and Final Decomposition Temperature (FDT) of native and modified LLSP was estimated with thermo grams.
Reusability of the modified LLSP
Desorption studies (batch process) were conducted to regenerate the biosorbent as a function of concentration of different desorption reagents: hard acid [0.05 M HNO3] and soft acid [0.5 M Citric acid]. Arsenic-loaded biosorbent obtained from our sorption experiments were transferred to Erlenmeyer flasks and shaken with 50 mL of each desorption reagents as a function of time (20, 40, 60, and 80 min) at room temperature. At the end of each time interval the suspension was stirred for 5 min. The suspension was filtered using Whatman 42 filter paper and in the filtrate estimation of metal ion concentration was carried out.
Statistical analysis
Batch experiments were conducted in triplicates (N = 3) and data represent the mean value. Correlation coefficient and standard deviations were calculated using SPSS PC +TM statistical package. For the determination of inter-group mean value differences, each parameter was subjected to the student t test for significance level (p < 0.05).
Results and discussions
Sorption efficiency of native LLSP
A series of experiments on sorption led to the standardization of the optimum conditions for arsenite (81.88 %) and arsenate (92.61 %) removal: arsenic concentration (25 μg/mL), contact time (40 min), volume (200 mL) and pH 7.5 for As (III) and 2.5 for As (V).
Effect of dosage
Biomaterial dosage used for the study varied from 2.0 to 6.0 g. Per cent sorption increased with the increase of biomaterial dosage from 2.0 to 4.0 g. However, no significant increment in the sorption tendency was observed on further increasing the biomaterial dosage from 4.0 g onwards. This might be due to attainment of equilibrium between adsorbate and adsorbent at the existing operating conditions rendering adsorbent incapable of further adsorption.
Effect of contact time
The effect of contact time on As (III) and As (V) sorption on modified LLSP was studied for the duration of 10–60 min. The per cent sorption of both the arsenic species gradually increased with time from 10 to 40 min, reaching to the optimum value. Once equilibrium was attained, the percentage sorption of both the arsenic species did not change with further increase of time.
Effect of arsenic concentration
Sorption behaviour of arsenic on plant biomaterial has been carried out in the range of arsenic concentration (0.05–50 μg/mL). Sorption of As (III) and As (V) on the biomaterial increased with increasing concentration of the arsenic reaching to an optimal level (25 μg/mL) and after that per cent sorption remained constant. These observations can be explained by the fact that at medium concentrations, the ratio of sorptive surface area to arsenic available is high, and thus there is a greater chance for arsenic removal. When arsenic concentrations are increased, binding sites become more quickly saturated as the amount of biomaterial concentration remained constant.
Effect of pH
The sorption potential of LLSP for As (III) and As (V) has been studied in the pH range of 2–10 (Fig. 1). The biosorption of As (III) shows gradually increasing trend, attaining maximum sorption (81.88 %) at pH 7.5. The biosorption of As (V) is maximum (92.61 %) at pH 2.5, while remaining constant in the pH range of 2.5–10.
Based on our experimental findings and relevant literature, we synthesize a possible mechanism for arsenic binding to the seed biosorbent. The seed powder of LLSP possesses various organic chemical moieties prominently large proportions of low-molecular-weight amino acids, having the ability to interact with metal ions (Costa et al. 1997). The trivalent arsenic species exists in a non-ionic form (H3AsO3) in the pH range 2–7 and in the anionic (H2AsO −13 , HAsO −23 ) form (Ghimire et al. 2002) in the pH range 7–10. Optimum sorption of arsenite species in the pH range 7–10 can be assigned to the availability of the negatively charged As (III) species interacting with the positively charged ends of amino acids, present in the target biosorbent, which have isoelectric points in the range 4–8 (Samuel 2002). However, sorption in the pH range 2–7 might be an affinity sorption process. The pentavalent arsenic species exists in the monovalent (H3AsO −14 ) and divalent anion (H2AsO42−) in the pH range 2–9. At lower pH the biosorbent is positively charged due to the protonation of amino groups. The negatively charged arsenate species may be held by the positively charged group of amino acids.
Enhancement in sorption efficiency of modified LLSP
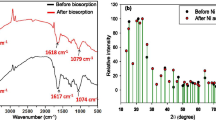
Functional group (amino) of the native LLSP has been enhanced using standard practises. Modified LLSP has been characterized. The FTIR spectra of native LLSP shows broad and strong bands in the range of 3,200 to 3,600 cm−1 due to the overlapping of OH and NH stretching bands (Fig. 2a), thus showing the presence of hydroxyl and amine groups on the biosorbent surface. Peaks appear in native LLSP at1744.9, 1,638.8 and 1,542.3 cm−1 can be assigned to COO–, C=O stretching and N–H bending, respectively. Shifting of band from 3,346.3 to 3,485.7 cm−1 has been found in the FTIR spectra of modified LLSP because of a large number of amine groups were introduced on the surface of the LLSP. The disappearance of the ester peak in PEI modified LLSP at 1,744.9 cm−1 may be related to a large number of the PEI macromolecules on the LLSP surface, which rendered the ester group undetectable in the FTIR analysis (Fig. 2b). Shifting of peaks of C=O stretching and N–H have been observed from 1,638.8–1,656.3 cm−1 to 1,542.3–1549 cm−1, respectively.
Modification on the LLSP can be further supported by the record of SEM image of native and modified LLSP. SEM of the native biosorbent exhibits large spherical clusters while that of modified biosorbent represents dense, agglomerated, irregular type morphology (Fig. 3). Modified biosorbent shows reduction in the pore area (native LLSP: 9.12 μm2 and modified LLSP: 2.30 μm2).
TGA of native and modified LLSP showed significant differences in the Final Decomposition Temperature (FDT) from 816 to 893 °C (Fig. 4).
The modified LLSP were measured for enhancement in the sorption efficiency for arsenic species under previously standardized optimum conditions. Significant increased in sorption efficiency in the modified LLSP has been observed with the same amount of dosage of biosorbent (4 g). The percentage sorption of As (III) increases from 81.88 to 85 %, while percentage sorption of As (V) increases from 92.61 to 99 % in modified LLSP (Table 1). The greater enhancement of percentage sorption of As (V) compared with As (III) species may be ascribed to the fact that As (III) has less chemical affinity with amino groups, compared with As (V), which shows higher affinity for amino groups (Spuches et al. 2005).
The reusability cycles of the modified LLSP were found to be increased up to 5 cycles, compared with 3 cycles of native LLSP. The increase in the reusable cycle in the case of modified LLSP is associated with their enhanced stability. The difference in decomposition temperature of native and modified LLSP shows the enhancement of its stability due to decrease in the weight loss of the biosorbent. Modifications using PEI have been found to form non toxic polymerized product (Lee et al. 2011). PEI itself is toxic in nature but resulting polymerized product is not, because of the neutralization of the positive charge on polyethyleneimine during the polymerization.
Conclusion
The laboratory-based findings open up new avenues in the abatement of arsenic species using LLSP which is nontoxic, biodegradable and available at an extremely low cost. The incorporation of positively charged amino groups in PEI modification enhanced the binding capacity of the biosorbents for negatively charged arsenic species. Modification leads to the enhancement in % sorption of As (III) from 81.88 to 85 % and As (V) from 92.61 to 99 %. However, the proposed method does not remediate arsenic species to the safe limit prescribed as 0.01 mg/L. It introduces a less expensive, domestic and environment-friendly pre treatment green method prior to high-tech chemical treatments. The reusability cycles of the PEI modified LLSP were found to be increased up to 5 cycles, compared with 3 cycles of native LLSP. The increase in the reusable cycle in the case of modified LLSP is associated with their enhanced stability.
References
Alvarado S, Guédez M, Lué-Merú MP, Nelson G, Alvaro A, Jesús AC, Gyula Z (2008) Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Biores Tech 99:8436–8440
Anirudhan TS, Unnithan MR (2007) Arsenic (V) removal from aqueous solutions using an anion exchanger derived from coconut coir pith and its recovery. Chemosphere 66:60–66
Bose U, Rahman M, Alamgir M (2011) Arsenic toxicity and speciation analysis in ground water samples: a review of some techniques. Int J Chem Tech 3(1):14–25
Burriel M, Conde L, Arribas J, Hernandez M (eds) (2006) Quımica Analıtica Cualitativa, 18th edn. Editorial Paraninfo, SA
Choonga TSY, Chuaha TG, Robiaha Y, Koaya FLG, Azni I (2007) Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination 217:139–166
Costa G, Michant JC, Guckert G (1997) Amino acids exuded from cadmium concentrations. J Plant Nutr 20:883–900
Ghimire KN, Inoue K, Makino K, Miyajima T (2002) Adsorptive removal of arsenic using orange juice residue. Sep Sci Technol 37:2785–2799
Gonzaga MIS, Santos JAG, Ma LQ (2006) Arsenic phytoextraction and hyperaccumulation by fern species. Scientia Agricola 63:90–101
Kardam A, Raj KR, Arora JK, Srivastava MM, Srivastava S (2010) Artificial neural network modeling for sorption of cadmium from aqueous system by shelled moringa oleifera seed powder as an agricultural waste. JWARP 2:339–344
Kundu S, Gupta AK (2006) Investigations on the adsorption efficiency of iron oxide coated cement (IOCC) towards As (V)—kinetics, equilibrium and thermodynamic studies. Colloid Surf A 273:121–128
Lee MS, Kim MG, Jangl YL, Lee K, Kim TG, Kim SH, Park TG, Kim HT, Jeong JH (2011) Polyethylenimine-g-poly(lactic-co-glycolic acid) as non-toxic micelle-type carrier for gene delivery. Macromol Res 19(7):688–693
Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168:1–12
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mohan D, Pittman CU, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gomez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310:57–73
Nemade PD, Kadam AM, Oza GH, Dutta SM, Shankar SH (2007) Adsorption of arsenite arsenate from water by HFO. Ind J Environ Protect 27:296–302
Patil AK, Shrivastava VS (2010) Removal of Cu(II) Ions by Leucaena leucocephala (Subabul) Seed Pods from Aqueous Solutions. E J Chem 7(S1):S377–S385
Pereira MSS, Winter E, Guimaraes JR, Rath S, Fostier AH (2007) A simple voltammetric procedure for speciation and evaluation of As removal from water. Environ Chem Lett 5:137–141
Raj KR, Kardam A, Arora JK, Srivastava MM, Srivastava S (2010) Neural network modeling for Ni(II) removal from aqueous system using shelled moringa oleifera seed powder as an agricultural waste. JWARP 2:331–338
Ranjan D, Talat M, Hasan SH (2009) Rice polish: an alternative to conventional adsorbents for treating arsenic bearing water by up-flow column method. Indus Eng Chem Res 48:10180–10185
Rivas BL, Aguirre MC (2010) Removal of As (III) and As (V) by Tin (II) compounds. Water Res 44:5730–5739
Samuel D (2002) Amino acids and proteins, 1st edn. IVY Publishing House, New Delhi
Shao W, Li X, Cao Q, Luo F, Li J, Du Y (2008) Adsorption of arsenate and arsenite anions from aqueous medium by using metal(III)-loaded amberlite resins. Hydrometallurgy 91:138–143
Sohel N, Ake PL, Rahman M, Kim SP, Muhammad Y, Charlotte EE, Marie V (2009) Arsenic in drinking water and adult mortality: a population based cohort study in rural Bangladesh. Epidemiology 20:824–830
Spuches AM, Kruszyna HG, Rich AM, Wilcox DE (2005) Thermodynamics of the As (III)-thiol interaction: arsenite and monomethylarsenite complexes with glutathione, dihydroliooic acid and other thiol ligands. Inorg Chem 44:2964–2972
Wang JS, Wai CM (2004) Arsenic in drinking water—a global environmental problem. J Chem Educ 81:207–213
Yeneneh MA, Maitra S, Eldemerdash U (2011) Study on biosorption of heavy metals by modified lignocellulosic waste. J Appl Sci 11(21):3555–3562
Acknowledgments
The authors gratefully acknowledge Prof. V.G. Das, Director and Prof. L.D. Khemani, Head, Department of Chemistry, Dayalbagh Educational Institute, Dayalbagh, Agra. They also gratefully acknowledge UGC [Project No.39-736/2010 (SR)] for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Raj, K.R., Kardam, A. & Srivastava, S. PEI modified Leucaena leucocephala seed powder, a potential biosorbent for the decontamination of arsenic species from water bodies: bioremediation. Appl Water Sci 3, 327–333 (2013). https://doi.org/10.1007/s13201-012-0057-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0057-y