Abstract
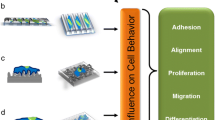
Tissue engineering requires tight control of stem cell function. Among many physical signals such as stretch and perfusion, geometrical cues have received much attention and have widely been recognized as an important factor in scaffold design. Here we review a variety of approaches that control stem cell fate at different levels of strictness, including micro-contact printing, microwells, direct cell printing, grooves, aligned micro-/nano-fibers, nanotubes, nanodots, hydrogel shape, and porous structure of scaffolds. Mechanical forces and signal transductions are discussed for cell shape regulated stem cell fate. Although many questions have yet to be resolved, geometry–force control is becoming an effective approach for the regulation of stem cell renewal and differentiation.



Similar content being viewed by others
References
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926.
Skalak, R., & Fox, C. F. (Eds.). (1998). Tissue Engineering: Proceeding of a workshop held at Granlibakken, Lake Tahoe, California. New York: Liss.
Frohlich, M., Grayson, W. L., Wan, L. Q., Marolt, D., Drobnic, M., Vunjak-Novakovic, G. (2008). Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Current Stem Cell Research & Therapy, 3(4), 254–264.
Jaenisch, R., & Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics, 33(Suppl), 245–254.
Guilak, F., Cohen, D. M., Estes, B. T., Gimble, J. M., Liedtke, W., Chen, C. S. (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell, 5(1), 17–26.
Thompson, D. A. W. (1943). On growth and form. Cambridge: University Press.
Nogawa, H., Morita, K., Cardoso, W. V. (1998). Bud formation precedes the appearance of differential cell proliferation during branching morphogenesis of mouse lung epithelium in vitro. Developmental Dynamics, 213(2), 228–235.
Mammoto, T., & Ingber, D. E. (2010). Mechanical control of tissue and organ development. Development, 137(9), 1407–1420.
Patwari, P., & Lee, R. T. (2008). Mechanical control of tissue morphogenesis. Circulation Research, 103(3), 234–243.
Ingber, D. E. (2006). Mechanical control of tissue morphogenesis during embryological development. The International Journal of Developmental Biology, 50(2–3), 255–266.
Adamo, L., Naveiras, O., Wenzel, P. L., McKinney-Freeman, S., Mack, P. J., Gracia-Sancho, J., Suchy-Dicey, A., Yoshimoto, M., Lensch, M. W., Yoder, M. C., Garcia-Cardena, G., Daley, G. Q. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature, 459(7250), 1131–1135.
Hove, J. R., Koster, R. W., Forouhar, A. S., Acevedo-Bolton, G., Fraser, S. E., Gharib, M. (2003). Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature, 421(6919), 172–177.
Auman, H. J., Coleman, H., Riley, H. E., Olale, F., Tsai, H. J., Yelon, D. (2007). Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biology, 5(3), e53.
Stevens, M. M., & George, J. H. (2005). Exploring and engineering the cell surface interface. Science, 310(5751), 1135–1138.
Ingber, D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. The FASEB Journal, 20(7), 811–827.
Hook, A. L., Voelcker, N. H., Thissen, H. (2009). Patterned and switchable surfaces for biomolecular manipulation. Acta Biomaterialia, 5(7), 2350–2370.
Kolind, K., Leong, K. W., Besenbacher, F., Foss, M. (2012). Guidance of stem cell fate on 2D patterned surfaces. Biomaterials, 33(28), 6626–6633.
Thery, M. (2010). Micropatterning as a tool to decipher cell morphogenesis and functions. Journal of Cell Science, 123(Pt 24), 4201–4213.
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M., Ingber, D. E. (1997). Geometric control of cell life and death. Science, 276(5317), 1425–1428.
Kilian, K. A., Bugarija, B., Lahn, B. T., Mrksich, M. (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 4872–4877.
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., Chen, C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell, 6(4), 483–495.
Wan, L. Q., Kang, S. M., Eng, G., Grayson, W. L., Lu, X. L., Huo, B., Gimble, J., Guo, X. E., Mow, V. C., Vunjak-Novakovic, G. (2010). Geometric control of adult human stem cell morphology and differentiation. Integrative Biology, 2(7–8), 346–353.
Vunjak-Novakovic, G. (2008). Patterning stem cell differentiation. Cell Stem Cell, 3(4), 362–363.
Luo, W., Jones, S. R., Yousaf, M. N. (2008). Geometric control of stem cell differentiation rate on surfaces. Langmuir, 24(21), 12129–12133.
Li, B., Li, F., Li, H.-X., Xu, X.-C., Szczodry, M., Yang, Z.-C., Lin, J.-S., Wang, J. H.-C. (2006). Cellular mechanical stress gradient regulates cell proliferation and differentiation patterns. Molecular & Cellular Biomechanics, 3(4), 225–227.
Ruiz, S. A., & Chen, C. S. (2008). Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells, 26(11), 2921–2927.
Munoz-Pinto, D. J., Qu, X., Bansal, L., Hayenga, H. N., Hahn, J., Hahn, M. S. (2012). Relative impact of form-induced stress vs. uniaxial alignment on multipotent stem cell myogenesis. Acta Biomaterialia, 8(11), 3974–3981.
Hwang, Y. S., Chung, B. G., Ortmann, D., Hattori, N., Moeller, H. C., Khademhosseini, A. (2009). Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proceedings of the National Academy of Sciences of the United States of America, 106(40), 16978–16983.
Moeller, H. C., Mian, M. K., Shrivastava, S., Chung, B. G., Khademhosseini, A. (2008). A microwell array system for stem cell culture. Biomaterials, 29(6), 752–763.
Park, J., Cho, C. H., Parashurama, N., Li, Y., Berthiaume, F., Toner, M., Tilles, A. W., Yarmush, M. L. (2007). Microfabrication-based modulation of embryonic stem cell differentiation. Lab on a Chip, 7(8), 1018–1028.
Karp, J. M., Yeh, J., Eng, G., Fukuda, J., Blumling, J., Suh, K. Y., Cheng, J., Mahdavi, A., Borenstein, J., Langer, R., Khademhosseini, A. (2007). Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab on a Chip, 7(6), 786–794.
Bauwens, C. L., Song, H., Thavandiran, N., Ungrin, M., Masse, S., Nanthakumar, K., Seguin, C., Zandstra, P. W. (2011). Geometric control of cardiomyogenic induction in human pluripotent stem cells. Tissue Engineering. Part A, 17(15–16), 1901–1909.
Peerani, R., Rao, B. M., Bauwens, C., Yin, T., Wood, G. A., Nagy, A., Kumacheva, E., Zandstra, P. W. (2007). Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO Journal, 26(22), 4744–4755.
Bauwens, C. L., Peerani, R., Niebruegge, S., Woodhouse, K. A., Kumacheva, E., Husain, M., Zandstra, P. W. (2008). Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells, 26(9), 2300–2310.
Schiele, N. R., Corr, D. T., Huang, Y., Raof, N. A., Xie, Y., Chrisey, D. B. (2010). Laser-based direct-write techniques for cell printing. Biofabrication, 2(3), 032001.
Ringeisen, B. R., Othon, C. M., Barron, J. A., Young, D., Spargo, B. J. (2006). Jet-based methods to print living cells. Biotechnology Journal, 1(9), 930–948.
Xu, T., Gregory, C. A., Molnar, P., Cui, X., Jalota, S., Bhaduri, S. B., Boland, T. (2006). Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials, 27(19), 3580–3588.
Roth, E. A., Xu, T., Das, M., Gregory, C., Hickman, J. J., Boland, T. (2004). Inkjet printing for high-throughput cell patterning. Biomaterials, 25(17), 3707–3715.
Raof, N. A., Schiele, N. R., Xie, Y., Chrisey, D. B., Corr, D. T. (2011). The maintenance of pluripotency following laser direct-write of mouse embryonic stem cells. Biomaterials, 32(7), 1802–1808.
Koch, L., Kuhn, S., Sorg, H., Gruene, M., Schlie, S., Gaebel, R., Polchow, B., Reimers, K., Stoelting, S., Ma, N., Vogt, P. M., Steinhoff, G., Chichkov, B. (2010). Laser printing of skin cells and human stem cells. Tissue Engineering. Part C, Methods, 16(5), 847–854.
Lee, W., Pinckney, J., Lee, V., Lee, J. H., Fischer, K., Polio, S., Park, J. K., Yoo, S. S. (2009). Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport, 20(8), 798–803.
Gerecht, S., Bettinger, C. J., Zhang, Z., Borenstein, J. T., Vunjak-Novakovic, G., Langer, R. (2007). The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials, 28(28), 4068–4077.
Yim, E. K., Darling, E. M., Kulangara, K., Guilak, F., Leong, K. W. (2010). Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials, 31(6), 1299–1306.
Wang, Y., Jiang, X. L., Yang, S. C., Lin, X., He, Y., Yan, C., Wu, L., Chen, G. Q., Wang, Z. Y., Wu, Q. (2011). MicroRNAs in the regulation of interfacial behaviors of MSCs cultured on microgrooved surface pattern. Biomaterials, 32(35), 9207–9217.
Mattotti, M., Alvarez, Z., Ortega, J. A., Planell, J. A., Engel, E., Alcantara, S. (2012). Inducing functional radial glia-like progenitors from cortical astrocyte cultures using micropatterned PMMA. Biomaterials, 33(6), 1759–1770.
Christopherson, G. T., Song, H., Mao, H. Q. (2009). The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials, 30(4), 556–564.
Li, W. J., Tuli, R., Okafor, C., Derfoul, A., Danielson, K. G., Hall, D. J., Tuan, R. S. (2005). A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials, 26(6), 599–609.
Nur, E. K. A., Ahmed, I., Kamal, J., Schindler, M., Meiners, S. (2006). Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells, 24(2), 426–433.
Yang, F., Murugan, R., Wang, S., Ramakrishna, S. (2005). Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials, 26(15), 2603–2610.
Xie, J., Willerth, S. M., Li, X., Macewan, M. R., Rader, A., Sakiyama-Elbert, S. E., Xia, Y. (2009). The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials, 30(3), 354–362.
Oh, S., Brammer, K. S., Li, Y. S., Teng, D., Engler, A. J., Chien, S., Jin, S. (2009). Stem cell fate dictated solely by altered nanotube dimension. Proceedings of the National Academy of Sciences of the United States of America, 106(7), 2130–2135.
Sridharan, I., Kim, T., Wang, R. (2009). Adapting collagen/CNT matrix in directing hESC differentiation. Biochemical and Biophysical Research Communications, 381(4), 508–512.
Chao, T. I., Xiang, S., Chen, C. S., Chin, W. C., Nelson, A. J., Wang, C., Lu, J. (2009). Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochemical and Biophysical Research Communications, 384(4), 426–430.
Huang, Y. J., Wu, H. C., Tai, N. H., Wang, T. W. (2012). Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small, 8(18), 2869–2877.
Mooney, E., Mackle, J. N., Blond, D. J., O’Cearbhaill, E., Shaw, G., Blau, W. J., Barry, F. P., Barron, V., Murphy, J. M. (2012). The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials, 33(26), 6132–6139.
Gadegaard, N., Thoms, S., Macintyre, D. S., McGhee, K., Gallagher, J., Casey, B., Wilkinson, C. D. W. (2003). Arrays of nano-dots for cellular engineering. Microelectronic Engineering, 67–68, 162–168.
Dalby, M. J., Gadegaard, N., Tare, R., Andar, A., Riehle, M. O., Herzyk, P., Wilkinson, C. D., Oreffo, R. O. (2007). The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials, 6(12), 997–1003.
Curran, J. M., Stokes, R., Irvine, E., Graham, D., Amro, N. A., Sanedrin, R. G., Jamil, H., Hunt, J. A. (2010). Introducing dip pen nanolithography as a tool for controlling stem cell behaviour: unlocking the potential of the next generation of smart materials in regenerative medicine. Lab on a Chip, 10(13), 1662–1670.
Lee, M. R., Kwon, K. W., Jung, H., Kim, H. N., Suh, K. Y., Kim, K., Kim, K. S. (2010). Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials, 31(15), 4360–4366.
Nayak, T. R., Andersen, H., Makam, V. S., Khaw, C., Bae, S., Xu, X., Ee, P. L., Ahn, J. H., Hong, B. H., Pastorin, G., Ozyilmaz, B. (2011). Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano, 5(6), 4670–4678.
Sant, S., Hancock, M. J., Donnelly, J. P., Iyer, D., Khademhosseini, A. (2010). Biomimetic gradient hydrogels for tissue engineering. Canadian Journal of Chemical Engineering, 88(6), 899–911.
Oh, S. H., Kim, T. H., Lee, J. H. (2011). Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials, 32(32), 8254–8260.
Wang, X., Wenk, E., Zhang, X., Meinel, L., Vunjak-Novakovic, G., Kaplan, D. L. (2009). Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. Journal of controlled release: official journal of the Controlled Release Society, 134(2), 81–90.
Groeneveld, E. H., & Burger, E. H. (2000). Bone morphogenetic proteins in human bone regeneration. European Journal of Endocrinology, 142(1), 9–21.
Lissenberg-Thunnissen, S. N., de Gorter, D. J., Sier, C. F., Schipper, I. B. (2011). Use and efficacy of bone morphogenetic proteins in fracture healing. International Orthopaedics, 35(9), 1271–1280.
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A., Bissell, M. J. (2006). Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science, 314(5797), 298–300.
Bian, W., Juhas, M., Pfeiler, T. W., Bursac, N. (2012). Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Engineering. Part A, 18(9–10), 957–967.
Carletti, E., Motta, A., Migliaresi, C. (2011). Scaffolds for tissue engineering and 3D cell culture. Methods in Molecular Biology, 695, 17–39.
Liu, X., & Ma, P. X. (2004). Polymeric scaffolds for bone tissue engineering. Annals of Biomedical Engineering, 32(3), 477–486.
Seunarine, K., Gadegaard, N., Tormen, M., Meredith, D. O., Riehle, M. O., Wilkinson, C. D. (2006). 3D polymer scaffolds for tissue engineering. Nanomedicine (London, England), 1(3), 281–296.
Karageorgiou, V., & Kaplan, D. (2005). Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials, 26(27), 5474–5491.
Hutmacher, D. W. (2001). Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. Journal of Biomaterials Science, Polymer Edition, 12(1), 107–124.
Kumar, G., Tison, C. K., Chatterjee, K., Pine, P. S., McDaniel, J. H., Salit, M. L., Young, M. F., Simon, C. G., Jr. (2011). The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials, 32(35), 9188–9196.
Smith, L. A., Liu, X., Hu, J., Ma, P. X. (2010). The enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials, 31(21), 5526–5535.
Kumar, G., Waters, M. S., Farooque, T. M., Young, M. F., Simon, C. G., Jr. (2012). Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials, 33(16), 4022–4030.
Bidan, C. M., Kommareddy, K. P., Rumpler, M., Kollmannsberger, P., Brechet, Y. J., Fratzl, P., Dunlop, J. W. (2012). How linear tension converts to curvature: geometric control of bone tissue growth. PLoS One, 7(5), e36336.
Graziano, A., D’Aquino, R., Cusella-De Angelis, M. G., Laino, G., Piattelli, A., Pacifici, M., De Rosa, A., Papaccio, G. (2007). Concave pit-containing scaffold surfaces improve stem cell-derived osteoblast performance and lead to significant bone tissue formation. PLoS One, 2(6), e496.
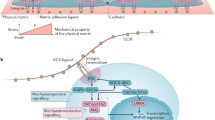
Vogel, V., & Sheetz, M. (2006). Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology, 7(4), 265–275.
Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126(4), 677–689.
Solon, J., Levental, I., Sengupta, K., Georges, P. C., Janmey, P. A. (2007). Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophysical Journal, 93(12), 4453–4461.
Discher, D. E., Janmey, P., Wang, Y. L. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science, 310(5751), 1139–1143.
Gjorevski, N., & Nelson, C. M. (2010). Endogenous patterns of mechanical stress are required for branching morphogenesis. Integrative Biology, 2(9), 424–434.
Raghavan, S., Shen, C. J., Desai, R. A., Sniadecki, N. J., Nelson, C. M., Chen, C. S. (2010). Decoupling diffusional from dimensional control of signaling in 3D culture reveals a role for myosin in tubulogenesis. Journal of Cell Science, 123(Pt 17), 2877–2883.
Cukierman, E., Pankov, R., Yamada, K. M. (2002). Cell interactions with three-dimensional matrices. Current Opinion in Cell Biology, 14(5), 633–639.
Cukierman, E., Pankov, R., Stevens, D. R., Yamada, K. M. (2001). Taking cell-matrix adhesions to the third dimension. Science, 294(5547), 1708–1712.
Fraley, S. I., Feng, Y., Giri, A., Longmore, G. D., Wirtz, D. (2012). Dimensional and temporal controls of three-dimensional cell migration by zyxin and binding partners. Nature Communications, 3, 719.
Doyle, A. D., Wang, F. W., Matsumoto, K., Yamada, K. M. (2009). One-dimensional topography underlies three-dimensional fibrillar cell migration. The Journal of Cell Biology, 184(4), 481–490.
Tan, J. L., Tien, J., Pirone, D. M., Gray, D. S., Bhadriraju, K., Chen, C. S. (2003). Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America, 100(4), 1484–1489.
Dembo, M., & Wang, Y. L. (1999). Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal, 76(4), 2307–2316.
Franck, C., Maskarinec, S. A., Tirrell, D. A., Ravichandran, G. (2011). Three-dimensional traction force microscopy: a new tool for quantifying cell-matrix interactions. PLoS One, 6(3), e17833.
Nelson, C. M., Jean, R. P., Tan, J. L., Liu, W. F., Sniadecki, N. J., Spector, A. A., Chen, C. S. (2005). Emergent patterns of growth controlled by multicellular form and mechanics. Proceedings of the National Academy of Sciences of the United States of America, 102(33), 11594–11599.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Worley, K., Certo, A. & Wan, L.Q. Geometry–Force Control of Stem Cell Fate. BioNanoSci. 3, 43–51 (2013). https://doi.org/10.1007/s12668-012-0067-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-012-0067-0