Abstract
At the Kristineberg mine, northern Sweden, sulphidic mine tailings were remediated in an 8-year pilot-scale experiment using sewage sludge to evaluate its applicability as a sealing layer in a composite dry cover. Sediment, leachate water, and pore gas geochemistry were collected in the aim of determining if the sludge was an effective barrier material to mitigate acid rock drainage (ARD) formation. The sludge was an effective barrier to oxygen influx as it formed both a physical obstruction and functioned as an organic reactive barrier to prevent oxygen to the underlying tailings. Sulphide oxidation and consequential ARD formation did not occur. Sludge-borne trace elements accumulated in a reductive, alkaline environment in the underlying tailings, resulting in an effluent drainage geochemistry of Cd, Cu, Pb and Zn below 10 μg/L, high alkalinity (810 mg/L) and low sulphate (38 mg/L). In contrast, the uncovered reference tailings received a 0.35-m deep oxidation front and typical ARD, with dissolved concentrations of Cd, Zn and sulphate, 20.8 μg/L, 16,100 μg/L and 1,390 mg/L, respectively. Organic matter degradation in the sludge may be a limiting factor to the function of the sealing layer over time as 85 % loss of the organic fraction occurred over the 8-year experimental period due to aerobic and anaerobic degradation. Though the cover may function in the short to medium term (100 years), it is unlikely to meet the demands of a long-term remedial solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preventative remedial measures against acid rock drainage (ARD) formation in tailings repositories have been extensively studied globally (INAP 2009; Lottermoser 2010) and within Sweden (Höglund et al. 2005). ARD is the result of the bacterially mediated natural weathering of pyrite (FeS2) and pyrrhotite (Fe(1−x)S) due to exposure to atmospheric oxygen and water. Typical ARD is characterised by elevated trace metal (Cd–Cu–Pb–Zn), sulphate, Fe and acidity loadings within the ground and surface waters of peripheral environments to tailings repositories, which may hinder surrounding environments. The focus on ARD mitigation has been either to neutralise the ARD in the far-field or to cover the tailings in the near-field to reduce the ARD potential of recently deposited mine tailings. In Sweden, composite dry cover designs are considered a successful long-term, low maintenance remediation solution (INAP 2009). They consist of a protective layer (PL) and a sealing layer (SL). The function of the PL is to protect the integrity of the underlying SL from erosion, root penetration, freeze–thaw weathering and wetting/drying cycles (Carlsson 2002b; Koerner and Daniel 1997). The underlying SL should have a low hydraulic conductivity (<10−9 m/s), with the primary function of preventing oxygen diffusion and water infiltration to underlying sulphidic mine waste. Composite covers used in Sweden have resulted in a 99 % (Lundgren and Lindahl 1993) and 95 % reduction in oxygen diffusion and water infiltration, respectively, compared to pre-remedial conditions, reducing 99.8 % of metals transported from tailings repositories (Carlsson 2002b).
Due to elevated costs of locating, excavating and transporting materials used for a SL, there has been demand to use alternative materials such as organic wastes that originate from the paper, pulp, water, and municipal waste industries (Gotthardsson and Sundberg 2005; Granhagen 1998; Mácsik et al. 2003; Neuschütz et al. 2009). One such organic waste is sewage sludge (SS) or biosolids which is the solid material generated during the treatment of domestic waste-water (Fytili and Zabaniotou 2008). In Sweden, approximately 210,000 tonnes of SS is produced annually (Neuschutz 2009), and regionally distributed from more than 2,100 waste-water treatment plants (Marklund 1997). In 2000, approximately 30 % of all SS in Sweden was used as surface covers for mine wastes (Wennman 2004). Since 2005, it has been prohibited from being land-filled (Ahlberg 2006) making it an attractive organic waste material to co-dispose with sulphide tailings.
Sewage sludge is not traditionally utilised as a SL material for mine waste, yet similar organic substrate applications, such as biosludge generated from the paper mill industry, have been used to cover mine waste in the long-term in Sweden (Hallberg et al. 2005). SS may have the potential for such usage because of its favourable physical and geochemical suitability (Wennman 2004; Ahlberg et al. 2006). The material content is variable between waste-water treatment plants but in general it has a low hydraulic conductivity of 1 × 10−9 m/s (Mácsik et al. 2003), high porosity of 90 % (Ahlberg 2006) and high water saturation potential of 95 % (Elliot et al. 1997), which is ideal for mitigating oxygen diffusion. In addition to the properties that may inhibit physical oxygen diffusion, fresh SS contains a high organic matter (OM) content that may allow it to function as an organic reactive barrier (Blowes et al. 2000).
Exposure of SS to atmospheric oxygen in surface layer applications has been found to result in rapid aerobic degradation and nitrification (Peppas et al. 2000). Applications may be more applicable as a subsurface SL, which would minimise dehydration and oxygen exposure, but that may be prone to anaerobic degradation processes such as methanogenesis and sulphate reduction. A combination of aerobic and anaerobic degradation processes in the SS may lead to rapid depletion of the OM fraction.
There is a lack of research focusing on sub-surface SS-SL’s buried beneath an overlying protective cover as part of an engineered composite dry cover used for the abatement of ARD. It is the aim of this study to determine if an application of SS as a SL in a composite cover is a viable long-term remedial solution to prevent ARD formation in sulphidic mine tailings. The objectives are to firstly quantify if the SS-SL is an effective barrier to oxygen diffusion, and consequential sulphide oxidation and ARD formation. Secondly to indicate and quantify dominant degradation processes in the SS and how these affect the function of the cover over time. Finally to understand the release, transport and attenuation of sludge- and tailings-borne trace elements. Data from pore gas, leachate and sediment geochemistry were derived from a pilot-scale experiment in northern Sweden over an 8-year time-span. They were compared to an uncovered reference tailings experiment. This study is the first pilot-scale experiment to assess the use of SS as a SL.
Experimental design
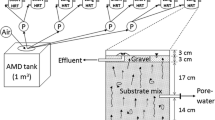
The Georange Environmental Test Site, located at the Boliden AB run Kristineberg Mine in northern Sweden, was completed in 2002. Two pilot-scale experimental test cells were used in this study (Fig. 1): an uncovered tailings control cell (UCC), and a sewage sludge remediated tailings cell (SSC). Each concrete cell measured 5 × 5 m2 by 3-m deep and was lined with an inert HDPE liner that resisted acid attack from the materials contained therein. The cells were insulated to prevent horizontal freezing and were open at the top to atmospheric conditions.
The bases of both cells were filled with a 0.3 m thick inert coarse-grained gravel layer to encourage water collection and drainage. In the SSC, the drainage layer was covered with a compacted 1.0-m thick fresh unoxidised tailings layer sourced from the Kristineberg Pb–Zn-Cu mine and processed at the nearby Boliden concentrator. A 1.4 m thick layer of uncovered tailings from the same source was applied in the UCC. The tailings in the SSC were additionally covered with a composite cover containing a sewage sludge sealing layer (SS-SL) (0.25 m), an upper drainage layer to mimic surface runoff (0.25 m), and a protective layer (PL) of locally derived glacial till (1.2 m).
The mineralogy of the tailings comprised the sulphides pyrite [FeS2] (48 %) and pyrrhotite [Fe(1−x)S] (4.8 %). The gangue minerals were quartz [SiO2], muscovite [KAl2(Si3AlO10)(OH)2], cordierite [Mg2Al4Si5O18], chlorite [(Mg, Fe)6(SiAl4O10)(OH)8], talc [Mg3Si4O10(OH)2], microcline [KAlSi3O8], diopside [Ca(Mg, Al)(Si, Al)2O6], K-feldspar [KAlSi3O8], and albite [NaAlSi3O8]. Calcite [CaCO3] and dolomite [CaMg(CO3)2] contents were 2.5 %, respectively, and during processing, approximately 10 kg/tonne of lime [Ca(OH)2] was added to the tailings (Alakangas 2006). The SS was derived from a nearby waste-water treatment plant in Lycksele (Fig. 1). After sedimentation of the raw sludge, aluminium-sulphate thickeners were added, and the SS was anaerobically digested. Both the tailings material and the SS contained elevated concentrations of Cd, Cu, Pb, and Zn (Table 1). The SS contained an OM content of 78 % of total solids (24 %).
Materials and methods
The solid materials were applied in August 2002 and sampling commenced in spring 2003, ending in summer 2010. This study is an extension of the data compiled from previous studies (Alakangas 2006; Alakangas and Öhlander 2006; Nason et al. 2010).
Water, sediment and gas sampling
Infiltrated leachate water derived from the base drainage of both cells was collected monthly from 2003 to 2006, and from 2009 to 2010 via probes in the drainage layer (1 × 1 × 0.2 m3) (Fig. 1). Data were devoid from 2007 to 2008 and in the winter months in sub-zero temperatures. The SSC contained a suction-lysimeter located immediately below the SS-SL and was collected during 2010.
Water for the analysis of dissolved major and minor elements was filtrated in the field using 0.22-μm Millipore nitrocellulose membrane filters that were acid-washed with 5 % acetate acid, and with syringes that were acid-washed using HNO3 then rinsed with Milli-Q® water. The particulate fraction was also analysed but not included. The dissolved fraction was transferred into 125 ml acid-rinsed bottles. Blank analysis using Milli-Q® water indicated a contribution of <2 % for all dissolved cations except for Pb which occasionally contributed concentrations exceeding 2 %. From 2009 to 2010, four separate samples were collected unfiltered into non-acid washed bottles for the anions NO3 −, SO4 2−, HCO3 − (alkalinity), total organic carbon (TOC), and dissolved organic carbon (DOC). In the field, all samples were stored in a cool, dark environment prior to analysis. Determinations of dissolved oxygen (DO), redox potential, pH, and temperature measurements were conducted. Measurements were performed using the following equipment: a Mettler-Toledo SevenGoPro dissolved oxygen metre, a Mettler-Toledo SevenGoPro Gmbh redox metre, a Metrohm® combined pH electrode from 2003 to 2006, and a Mettler-Toledo MP125 pH and temperature electrode from 2009 to 2010. Both were calibrated to standards of pH 4.01 and pH 7.00, respectively.
A sediment core was collected in February 2010 from the SSC using an Atlas Copco Cobra TT percussion hammer drill. Samples collected were extracted from a depth of 1.10–1.55 m and consisted of the SS and tailings layers. The samples were collected in a 0.05 m diameter core and extracted into non-diffusive plastic bags within an argon filled chamber. All sampling took place in an anoxic environment. Nine 0.05 m thick sub-samples totalling 0.15 m SS and 0.3 m tailings thicknesses were retrieved. Sediment samples derived from the UCC were collected in June 2009 using a mechanical excavator to expose a uniform tailings profile. A non-metallic trowel was used to extract the tailings into an argon filled chamber to avoid oxygen contamination. Eleven 0.1 m thick sub-samples totalling 1.10 m tailings were retrieved including a 0.02 m sample at the oxidation front. Samples were stored in a dark frozen environment and were not exposed to oxygen until sample analysis. The in situ solid pH was measured using a Mettler-Toledo pH electrode.
Two quartzite filled geotextile balls were installed in the SSC to collect oxygen, methane and carbon dioxide at 0.1 m above, 0.1, and 0.5 m below the SL (Fig. 1). Samples were extracted using a Maihak S710 gas analyser. The gas samples were analysed biweekly during spring, summer, and autumn in 2004 and once a month during 2005. Methane and carbon dioxide were calibrated with specific gas concentrations prior to the sampling. The precision of the instrument was better than 2 % of the analysed value, according to the manufacturer.
Chemical analysis and geochemical modelling
Dissolved concentrations of major (Ca, Fe, K, Mg, Na, S, Si, Al) and minor (As, Ba, Cd, Co, Cr, Cu, Mn, Mo, Ni, P, Pb, Zn) elements in the leachate were derived using ICP-AES and ICP-SFMS, respectively. Alkalinity was determined by titrating with hydrochloric acid while purging carbon to an endpoint pH of 5.4. Nitrate and sulphate were analysed using ion chromatography. The analyses were conducted according to the EPA methods (modified) 200.7 (ICP-AES) and 200.8 (ICP-SFMS).
The extraction of metals prior to analysis was performed by digesting the sediment after they were dried at 50 °C by leaching in 7 M nitric acid in closed Teflon vessels in a microwave oven for the elements As, Cd, Co, Cu, Hg, Ni, Pb, S and Zn. The remaining elements, Al, Cr, Fe and Zr, were extracted using fusion with lithium metaborate and dissolved in dilute nitric acid. These solutions were centrifuged and diluted before analysis. Analysis was conducted using ICP-AES for Al, Cr, Fe, S, and Zr using ICP-SFMS for Cd, Co, Cu, Hg, Ni, Pb and Zn. Instrumental analysis was carried out according to modified USEPA methods. Precision in all methods was generally better than 5 %.
Total organic carbon samples in the leachate water were calculated by determining particulate organic carbon (POC) and DOC fractions. Samples were filtered through 25-mm diameter glass micro fibre filters (0.47 μm) mounted in stainless steel filter holders. POC filters were stored in tin foil. Analysis of POC using a Carlo Erba Model 1108 high-temperature (1,030 °C) combusting element analyser was used, and DOC analysis was performed using a Shimadzu TOC-5000 high-temperature combustion instrument.
Organic complexation modelling, using the chemical equilibrium Visual MINTEQ modelling software (Version 3.0., Beta), was utilised to further enable an interpretation of the interaction between DOC concentrations and dissolved metal concentrations from the leachate derived from the SSC. The Stockholm Humic Model was used to model the dissolved leachate geochemical data (Gustafsson 2001).
Though a total elemental suite was analysed and modelled in this study, only the minor metal elements (Cd–Cu–Pb–Zn) related to primary ore minerals in the tailings and the major elements (Fe–S) related to geochemical reactions have been discussed.
Results and discussion
Efficiency of sewage sludge sealing layer
Visual inspection of the SS-SL upon sampling revealed little physical change since deposition. The layer remained a compact 0.15–0.25 m thick, black coloured layer with no indication of preferential water pathways, cracks, or desecration. Difference in thicknesses occurred due to the settling of the overlying till protective layer.
Evaluation of oxygen diffusion
Oxygen movement through a gas-filled porous medium, such as the SS-SL, is common by means of physical diffusive gas transport. Using a re-arrangement of Fick’s first law as a model for gas diffusion (Eq. 1), it is possible to calculate the amount of oxygen (F diff) (mol−1 m−2 year−1) that was able to pass through the SS-SL by knowing the difference in oxygen gradient across the SS-SL boundary (ΔC) as derived from the oxygen data in Fig. 2. This is presuming steady-state conditions. Using an estimation of a hydraulic conductivity of 1 × 10−9 m/s (Mácsik et al. 2003) for the SS, the D eff value was estimated at a saturation of 95 % (Elliot et al. 1997) using the Millington model (Carlsson 2002b). With a known thickness (ΔZ) of the SS-SL of 0.25 m, a calculated mass transfer of 0.54 mol−1 m−2 year−1 oxygen occurred during the period 2004–2005.
This value is below the limitations set out for a conventional sealing layer in Sweden of <1 mol−1 m−2 year−1 (Carlsson 2002a) and, is hence, a suitable barrier material. However, uncertainties surround the calculation of the D eff value due to uneven compaction of the sewage sludge, although care was taken upon application to avoid this. Nevertheless, Ahlberg (2006) suggested the sewage sludge layer would be more effective if it were fully water-saturated, which would impede oxidation of the cover material and improve the cover function and integrity over time.
Aerobic and anaerobic degradation in the sewage sludge
Measured oxygen, carbon dioxide, and methane (Fig. 2) concentrations from the SSC, both above and below the SL, indicate that the SS may have functioned as an organic reactive barrier. Looking at correlations between the gas concentrations above and below the SS-SL (Fig. 3), it is possible to identify processes indicative of organic degradation, both aerobically and anaerobically, that have reduced the OM (LOI) contents in the SS-SL. Using the original concentrations of LOI in the SS (Table 1), it is possible to calculate that in the upper, middle, and lower sections of the SS-SL, the organic matter contents became depleted by 42, 64.7 and 79.3 %, respectively, using the sample concentrations derived from Fig. 4.
Aerobic degradation of the OM fraction associated with the consumption of oxygen and the production of carbon dioxide occurred. The upper 0.05 m of the SS-SL was prevalently affected because of the interaction of high concentrations of atmospheric oxygen diffusing through the PL from above (Fig. 2). Oxygen contact promoted degradation of the SS-SL between late summer and early autumn when the SS was dehydrated and exposed to atmospheric oxygen. During late spring to early summer, and again in autumn, the SL was saturated due to the spring snow melt and autumn storm events, respectively. At these intervals in the year, aerobic degradation was negligible due to the low diffusivity of oxygen in water. In winter, from December to March, frozen conditions in the PL and SL occurred (Shcherbakova 2006) all of which may have somewhat limited the transport of oxygen (Elberling 2005). Consequently, oxygen was consumed in the upper SS-SL and concentrations in the underlying tailings were below 1 % year round (Fig. 2). Carbon dioxide, a product of this reaction, was negatively correlated to oxygen concentrations above the SL (Fig. 3: 1a) indicating that when aerobic degradation of OM occurred, CO2 was released as a product of the reaction. The base of the SS-SL received no aerobic degradation, as carbon dioxide and oxygen concentrations showed no correlation (Fig. 3: 2a).
Surface applications of SS have shown that aerobic degradation may account for an oxidation depth of 0.8 m depth in 4 years (Ahlberg 2006) producing shrinkage cracks and preferential flow development which may compromise the integrity of a SL. However, because the SL in the SSC was a compacted sub-surface layer and it was water-saturated or frozen for three-quarters of the year, similar structural disintegration did not occur. Aerobic degradation processes, resulting in organic matter depletion, were limited to the upper SS-SL only.
Anaerobic degradation of the OM fraction generates methane and carbon dioxide as products of the reaction. The layer must be water saturated and anoxic for anaerobic degradation and methanogenesis to occur (Markewitz et al. 2004). At the top of the SS-SL, methane and carbon dioxide concentrations were positively correlated in 2005 (Fig. 2: 1c) together with a negative correlation between oxygen with methane (Fig. 2: 1b). This allows the assumption that there was a development of water-saturated conditions in the SS-SL over time and that methanogenesis was initiated. At the base of the SS-SL, anaerobic degradation was also prevalent and initiated in 2005 with similar correlations between carbon dioxide and methane as at the top of the SS-SL (Fig. 3: 2c). Concentrations of methane were an order of magnitude higher below the SS-SL than above (Fig. 2). These concentrations were independent of oxygen concentrations (Fig. 3: 2b) which were an order of magnitude lower at the base (<1 %). Therefore, the environment within the SS-SL was anoxic, water-saturated and devoid of oxygen—an environment that was able to host anaerobic degradation processes. The magnitude of anaerobic degradation processes compared to aerobic degradation processes at the top of the SL was more elevated as it occurred continuously throughout the year, whereas aerobic degradation was limited to 25 % of the year. This accounted for the greater depletion in OM concentrations from the base of the SL than from the top.
A further indication that oxygen concentrations still remained low underneath the SL were identified from the redox measurements of the leachate, collected from 2003 to 2010, from the base drainage layers. Redox (pe) in the SSC measured 2.8 ± 0.6. In comparison, the redox in the base of the UCC measured 5.95 ± 3.57.
Release of trace elements from the sewage sludge
Quantification of element re-distribution within the solid fraction of the SS can be derived by normalising to a fixed element, such as Al, Si, Ti, or Zr, that may remain immobile during the weathering of solid material. Elements in the SS were normalised from the original SS compositions (Table 1) using Al2O3 as a substitute for the more preferable normalising elements Si, Ti, and Zr as these were not available in the original geochemical analysis. A minor risk of using this element is presented. Process flocculants added during the treatment of SS before the application contained Al. However, it is likely that these were largely flushed out before the experiment began and the Al used as a proxy is contained in the residual solid fraction. Mass balance calculations were performed at each sample interval using a modification of Gresens’ equation of metasomatic alteration (Grant 1986). The original concentration (C O) and the final concentration (C F) of a particular element (e) are expressed as ratios to a normalising element (n).
Elements released from the SS were preferentially depleted at the base of the SL (Fig. 4: 2a) compared with the top, where Pb and Zn were enriched by 28 and 4.6 %, respectively, and Cd and Cu were less depleted. At the base of the SL, Cd, Cu, Pb, and Zn were all depleted by 85, 80, 74, and 75 %, respectively. These elements were highly elevated in the original SS composition (Table 1). Ahlberg (2006) reported, in a separate study, that the removal of elevated trace element concentrations from SS was dominantly in the form of organic colloidal matter in the first 2 years of application affecting Cu and Pb, and that after this period with the accelerated degradation of OM in the SS, the elements Cd and Zn were lost as dissolved organo-metallic complexes associated with the release of DOC. In the SSC, this may have accounted for the removal of Cd, Cu, Pb, and Zn from the SS-SL base as OM degradation rates were higher. The pH in the SS-SL was within 7.2–7.5 (Fig. 4: 1h), which aided the efficiency of metal removal as organo-metallic complexes (Fletcher and Beckett 1987).
A reduction of OM in the SS-SL occurred as discussed earlier. The degradation was most prevalent at the sludge-to-tailings interface (Fig. 4: 1g) where 94 % of the OM had been lost since deposition when compared to the original OM content of SS. However, on average, the OM fraction had only been reduced by 85 % throughout the whole layer and was lowest in the top of the SS-SL (Fig. 4: 2a) with a 77 % total loss. OM degradation may be attributed to aerobic/anaerobic degradation processes.
Iron increased in the entire SS-SL which may have contributed mass enrichment (Fig. 4: 2b). This may have been derived from the migration of fine silt and sand from the overlying protective till material which is rich in relatively inert feldspar, clay, and silicate minerals. Total mass balance calculations of the SL indicate that the degradation of OM, with the loss of elements leached combined with the influx of constituents from the glacial till, totalled a mean mass loss of 19.6 % in the SL within the 8 years since application (Fig. 4: 2c). This 19.6 % mass loss contrasts to surface applications of SS, where a much higher 30 % mass volume reduction may occur within only a 4-year time frame (Ahlberg 2006). Hence, the mass reduction in a sub-surface SS-SL is 3 times lower than in a surface application.
Release, transport and attenuation of trace elements in tailings
Elements in the tailings underlying the SS-SL in the SSC and the tailings in the UCC were normalised using Zr as the normalising element due to the zirconium’s mineral (ZrSiO4) resistance to low-temperature weathering (Nickel 1973). Mass balance calculations were performed at each sample interval of both profiles using equations (2/3) and normalised to the unoxidised tailings geochemistry at the base of the UCC tailings profile.
Sewage sludge cell tailings
The tailings beneath the SS-SL contained an accumulation zone immediately below the sludge-to-tailings interface (Fig. 4: 1a–g). This 0.05 m thick zone contained a mass enrichment of 43, 41, 34, 57 %, and 62 % for, Cd, Cu, Pb, S, and Zn, respectively (Fig. 4: 2a–c). The pH data (Fig. 4: 1g) indicated that the metals precipitated at a pH of 7.3–7.5 in the tailings which likely caused one of the depositional controls. The metal accumulation zone was accompanied by an enrichment of OM of 54 % (Fig. 4: 2a) which conforms to correlations between metals and OM in previous studies (Abu-Zied et al. 2013). The organically bound metals sourced from the SS-SL, percolated down into the tailings and become trapped, likely accounting for the enrichment in Cd, Cu, Pb, and Zn. This accumulation conforms to similar research conducted within soil profiles (Ahlberg et al. 2006; Ashworth and Alloway 2004) where SS-derived metals accumulate immediately within underlying topsoil layers.
Sulphur and Fe2O3 were elevated in the original SS (Table 1) and accumulated in the tailings to a mass enrichment of 47 and 75 %, respectively. Bacterially mediated sulphate reduction may have occurred due to the anaerobic environment created beneath the SS-SL (Herbert et al. 2000), which may have further contributed to anaerobic degradation processes in the SS-SL. Sulphate flocculants added during sludge digestion in water treatment plants may have been reduced. However, no correlation between sulphur and Fe2O3 exists in the data which dismisses the formation of secondary sulphide precipitation. However, sulphur and Cd–Cu–Pb–Zn have been correlated in the data (R 2 > 0.98) underlying the SS-SL. It is likely that secondary metal-sulphate minerals may have precipitated. The mass balance calculations indicate that metals accumulated in the tailings were derived from the SS and immobilised in the tailings due to governing geochemical controls. Metals released due to oxidation of the sulphide-bearing mine tailings by oxygen diffusion through the SS-SL are not evident.
Uncovered control cell tailings
The UCC tailings profile displayed a typical formation of an observed oxidised zone showing a 13.88 % total mass loss of elements in the upper 0.35 m tailings (Fig. 5: 2C). Weathering was prevalent at the surface where oxygen concentrations were within 15 % between 2004 and 2005 (Alakangas et al. 2012). Fe2O3, Cd, Cu, Pb, S, and Zn were released from the oxidised zone by 10, 24, 5, 48, 39, 13%, respectively (Fig. 5: 2a, b). An acute boundary between the oxidised weathered tailings zone and the underlying unoxidised tailings was identified by the acidic front formation with a signature low pH (Fig. 5: 1g) and, in addition, the elements Cd, Cu, and Zn, mobile in this low pH zone, were depleted, whereas Pb remained immobile (Fig. 5: 1a–f). The low pH at the acidic front is attributed to the dissolution of pyrite/pyrrhotite in an oxidised environment. The trace elements depleted from the oxidised zone are likely derived from dissolution of the trace sulphides in the tailings due to the low pH, such as sphalerite and chalcopyrite, or the presence of these elements in the crystal lattices of pyrite or pyrrhotite. A relative enrichment of the residual fraction remained in the oxidised zone. Immediately beneath the acidic front, an increase in pH promoted an accumulation of the mobilised constituents, within the unoxidised tailings, in a similar magnitude to what had been lost in the oxidised zone. It is likely that this precipitation occurred as secondary mineral precipitates. The overall mass balance (Fig. 5: 2c) illustrates this relationship. The data conform to previous models focused upon calculating oxidation rates in uncovered tailings within the 8-year period since deposition (Ritchie 1994) and have been modelled using a 1-D model PYROX on these data (Alakangas et al. 2012).
Quality of the drainage leachate
Concentrations of anions (Fig. 6) and dissolved elements (Fig. 7) derived from the UCC and SCC drainage leachate, together with the SSC lysimeter, are compared in this section. Furthermore, the drainage lysimeter located immediately below the SS-SL was collected in 2009–2010 to model the relationship of metals bound to dissolved organic matter (DOM) for Fe(II), Cu(II), Cd(II), Pb(II), and Zn(II) together with the base drainages of both cells. The model assumed DOC concentrations to contain 100 % fulvic acid at a ratio of 1.65, and it is important to note that the total dissolved carbon (TOC) was almost entirely composed of DOC (Fig. 6). The model was based on data obtained by taking the mean values from the base drainage probe and the lysimeter directly below the SL during the sampling period 2009–2010. Redox values were entered into the model from field measurements of redox potential (mV) re-calculated into pe. The redox couple Fe2+/Fe3+ was chosen. The model output ‘Bound to DOM’ was selected.
Diagram to illustrate mean 2009–2010 anion and dissolved oxygen (DO) concentrations between the drainage lysimeter located 0.1 m below the SS-SL in the SSC; the base drainage in the SSC; the base drainage in the UCC; at relative depths in the tailings profile. Note the UCC drainage layer is placed to scale and is actually 1.4 m below the surface
Sewage sludge cell lysimeter
Dissolved organic carbon concentrations inputted into the model were very high immediately below the SS-SL (215 mg/L). The model simulation estimated an increased affinity for metallic elements to bind to DOM as organic complexes. 100 % of Cu(II) ions were bound as strong organic-complexes with DOM. The metals Cd(II), Fe(II), Pb(II), and Zn(II) were also bound as strong organo-metallic complexes with the DOM by 81, 28, 99 and 74 %, respectively. This relationship between sludge-borne metals and DOC released indicates that metals were transported in association with OM in solute fractions, together with colloidal fractions as already discussed. The pH of the leachate was near-neutral, which allowed an increased attraction of cations to binding sites such as those possessed to carboxylic acids of DOM due to the dissociation of H+ ions and the formation of the strong binding organo-metallic complexes (Garcia-Gil et al. 2007). This explains the transport of elevated levels of metals from the SS-SL to the underlying tailings at a high pH.
Sewage sludge cell drainage
In 2003, high Fe–Cd–Cu–S–Zn concentrations were released from the tailings into the base drainage due to the flush of residues from the freshly processed tailings (Fig. 7). However, the magnitude and duration of this flush subsided within 1 year, and from 2005 to 2010, concentrations of Cd, Cu, Pb, and Zn were below 10 μg/L and sulphate concentrations varied between 39 and 105 mg/L (Fig. 6). Low sulphate concentrations were mirrored by elevated alkalinity concentrations (810 mg/L HCO3 −), which are further indicative of sulphate reduction by organic matter degradation. Furthermore, a reductive environment formed (Fig. 7). These data indicate that oxygen diffusion through the SS-SL was restricted, sulphide oxidation of the underlying tailings was deficient and ARD formation did not occur. Sludge-borne metals did not leach through the entire tailings profile to the effluent base drainage leachate either. They precipitated and were retained in the accumulation zone in the tailings immediately underlying the SS-SL. There was no evidence of sulphide oxidation in the tailings, as dissolved oxygen concentrations immediately underlying the SS-SL were <1 mg/L in the leachate, and DO concentrations in 2010 were below 1.5 mg/L in the base leachate (Fig. 6). This resulted in no further element release from the tailings.
The mean DOC concentration modelled was 17 mg/l (Fig. 6). The divalent Cd(II), Fe(II) and Zn(II) cations had high concentrations of free ions in solution and did not have a high affinity to complex with DOM. The divalent Cu(II) and Pb(II) cations formed moderate organo-metallic complexes to DOM by 69 and 27 %, respectively. The DOC and metal concentrations of these metals in solution were lower than immediately underlying the SS-SL, showing that metals and OM were retained in the tailings. The immobilisation processes that controlled metal precipitation in the accumulation zone at the sludge-to-tailings interface were most likely controlled by precipitation of organo-metallic complexes onto pre-existing Al- and Fe-(oxy)hydroxide residues in the tailings, such as the process that occurs in the B-horizon of a podzol soil. The transport of sludge-borne metals as organo-metallic complexes with the DOM accounted for the high elemental retention capacity of the tailings, creating a drainage geochemistry at the base of the SSC that was very low in concentrations of dissolved metals.
Uncovered control cell drainage
An initial flush caused by the fresh tailings residues of all elements occurred during the first year after deposition in 2003. Concentrations of the metals Cd, Cu, Pb, and Zn in the leachate decreased after the initial flush of constituents from 2003 to 2006, but began increasing thereafter due to the onset of sulphide oxidation. Exceptionally, high concentrations of Cd and Zn occurred by as much as 20.8 and 16,100 μg/L, respectively, in 2010, far exceeding the concentrations in the SSC drainage. The mobilisation control of these constituents is attributed to the acidity released in the oxidation front by continued pyrite oxidation as indicated by the high dissolved oxygen concentrations of 6 mg/L in the leachate (Fig. 6). In 2009, the mean SO4 2− values in the base leachate were 1,390 mg/L (Fig. 6).
With an elevated pH, this type of drainage is characterised as neutral mine drainage (NMD) (INAP 2009). Alkalinity (Fig. 6) concentrations in the UCC drainage leachate from 2003 to 2010 were relatively elevated with mean values of 88 mg/L. This was due to the dissolution of lime (Ca(OH)2), added during mineral processing, and calcite gangue minerals which reacted with acidity producing the NMD. However, due to the high (ca. 50 %) sulphide and the low (ca. 5 %) carbonate mineral content in the original tailings, it is likely that the pH will reduce over time due to continued acidity release from sulphide oxidation, which in turn, will exhaust the carbonates and begin to produce typical ARD.
Nitrification and nitrate as an oxidant
Initially, N is usually present as ammonium (NH4 +) in high concentrations in the SS (Neuschütz et al. 2009), and that over time, exposure to oxygen may release nitrate (NO3 −) from the process of nitrification. Nitrate is a possible oxidant for pyrite where oxygen is devoid, and may facilitate the release of elevated sulphate and Fe into solution. Both nitrification and pyrite oxidation promote acidification of tailings treated by surface applications of SS (Cravotta 1998; Stehouwer et al. 2006), which may elevate the amount of metals transported in solution from these application types. The rate of nitrification should decrease exponentially due to exhaustion of ammonium in the OM fraction of the SS (Evanylo 1994). Nitrate data were derived in 2009–2010 from the lysimeter and drainage leachate in the SSC (Fig. 6), but were devoid from 2003 to 2009. Low concentrations (<1 mg/L) of nitrate in the leachate underlying the SS-SL occurred. This increased marginally in the base leachate (6.5 mg/L). Though the values are within EU limits (<10 mg/l), and up to two orders of magnitude lower than nitrate concentrations from surface applications of SS where nitrification has occurred (Ahlberg 2006), they were higher in the SSC than the UCC cell base indicating nitrate had been released through the process of nitrification in the SS-SL. However, geochemical evidence of the occurrence of past elevated nitrification in the SS-SL is lacking as no acidification occurred in the SS or the underlying tailings. The impact of nitrate on the oxidation of sulphide minerals was minimal. Furthermore, low dissolved Fe, sulphate, and acidity concentrations in the SSC base leachate indicate that it is likely that nitrate was not an oxidant to pyrite in the tailings.
Conclusions
The use of SS as a SL material was effective at preventing oxygen diffusion to underlying sulphide-bearing mine tailings for an 8-year period. It was additionally advantageous in that it created a reductive, alkaline environment in the underlying sulphidic-mine tailings, with a high buffering capacity, which together promoted the precipitation of metals, removing them from solution and improving the quality of the effluent drainage. Without the SS-SL and PL, uncovered sulphide-bearing mine tailings received extensive sulphide oxidation characterised by the onset of ARD and a 0.35 m deep acidic oxidation front formation
Though the SS-SL was effective at preventing oxygen diffusion, sulphide oxidation and ARD formation to the underlying tailings and drainage, the integrity and function of the SL may have become compromised over time due to the loss of OM by degradation processes. Anaerobic degradation controlled the majority of OM degradation in the SS-SL, largely at the SL base, where oxygen was absent. Furthermore, aerobic degradation by oxygen at the top of the SL contributed to an OM mass depletion and the release of metals from the SS-SL. Nitrification and nitrate-oxidation of sulphides was minimal, contrasting with surface applications. The SS used in this study was anaerobically digested which has an increased OM content than aerobically digested SS, and may have affected the magnitude of degradation rates. In the 8 years since application, 85 % depletion in the OM fraction of the layer occurred, accounting for a total volume loss of 19.6 % from the SL. This a third less than surface applications of SS. Nevertheless, it should be considered a possible limiting factor when using a SS-SL in the long-term (>100 years), though in the experimental time period of this study, the sealing layer continued to function appropriately. The use of SS as a SL to remediate sulphidic mine tailings by the mining industry in Sweden is possible, though further quantification of the long-term applicability based upon the degradation rates of the OM fraction is required.
References
Abu-Zied RH, Basaham AS, El Sayed MA (2013) Effect of municipal wastewaters on bottom sediment geochemistry and benthic foraminifera of two Red Sea coastal inlets, Jeddah, Saudi Arabia. Environ Earth Sci 68:451–469
Ahlberg G (2006) Ageing of Sewage Sludge—some physical and chemical properties in relation to landscaping. Doctoral Dissertation, Göteborg University
Ahlberg G, Gustafsson O, Wedel P (2006) Leaching of metals from sewage sludge during one year and their relationship to particle size. Environ Pollut 144:545–553
Alakangas L (2006) Sulphide oxidation, oxygen diffusion and metal mobility in sulphide-bearing mine tailings in northern Sweden. Doctoral Dissertation, Luleå University of Technology
Alakangas L, Öhlander B (2006) Pilot Scale studies of different covers on unoxidised sulphide-rich tailings, northern Sweden: the geochemistry of leachate waters. Mine Water Environ 25:171–183
Alakangas L, Lundberg A, Nason P (2012) Simulation of pyrite oxidation in fresh mine tailings under near-neutral conditions. J Environ Monit 14:2245–2253
Ashworth DJ, Alloway BJ (2004) Soil mobility of sewage sludge-derived dissolved organic matter, copper, nickel and zinc. Environ Pollut 127:137–144
Blowes DW, Ptacek CJ, Benner SG, McRae CWT, Bennett TA, Puls RW (2000) Treatment of inorganic contaminants using permeable reactive barriers. J Contam Hydrol 45:123–137
Carlsson E (2002a) Soil-cover remediation of sulphide-rich tailings. Technical Report, SBLO50: EC2002
Carlsson E (2002b) Sulphide-rich tailings remediated by soil cover—evaluation of cover efficiency and tailings geochemistry, Kristineberg, northern Sweden. Doctoral Dissertation, Luleå University of Technology
Cravotta CA (1998) Effect of sewage sludge on formation of acidic ground water at a reclaimed coal mine. Ground Water 36:9–19
Elberling B (2005) Temperature and oxygen control on pyrite oxidation in frozen mine tailings. Cold Reg Sci Technol 41:121–133
Elliot LCM, Liu L, Stogran SW (1997) Organic Cover Materials for Tailings: Do they meet the requirements of an effective long term cover? In: Fourth International Conference in Acid Rock Drainage (4th ICARD), Vancouver, B.C. Canada
Evanylo GK (ed) (1994) Mineralisation and availability of nitrogen in organic waste-amended mid-Atlantic soils. Chesapeake Research Consortium Inc, Edgewater
Fletcher P, Beckett PHT (1987) The Chemistry of Heavy-Metals in Digested Sewage-Sludge-I. Copper(II) Complexation with Soluble Organic-Matter. Water Res 21:1153–1161
Fytili D, Zabaniotou A (2008) Utilization of sewage sludge in EU application of old and new methods—a review. Renew Sust Energy Rev 12:116–140
Garcia-Gil JC, Plaza C, Senesl N, Brunetti G, Polo A (2007) Effects of long-term sewage sludge amendment on the composition, structure and proton binding activity of soil fulvic acids. Clean Soil Air Water 35:480–487
Gotthardsson J, Sundberg Å (2005) Mine site reclamation using products from the pulp industry. In: Securing the Future: International Conference on Mining and the Environment, metals and energy recovery, Skellefteå, Sweden
Granhagen JR (1998) A fly-ash/biosludge dry cover for the mitigation of acid mine drainage. Licentiate Dissertation, Stockholm University
Grant JA (1986) The Isocon diagram—a simple solution to Gresens’ equation for metasomatic alteration. Econ Geol 81:1976–1982
Gustafsson JP (2001) Modeling the acid-base properties and metal complexation of humic substances with the Stockholm humic model. J Colloid Interface Sci 244:102–112
Hallberg RO, Granhagen JR, Liljemark A (2005) A fly ash/biosludge dry cover for the mitigation of AMD at the falun mine. Chem Der Erde Geochem 65:43–63
Herbert RB, Benner SG, Blowes DW (2000) Solid phase iron-sulfur geochemistry of a reactive barrier for treatment of mine drainage. Appl Geochem 15:1331–1343
Höglund LO, Herbert RB, Lövgren L, Öhlander B, Neretnieks I, Moreno L, Malmström M, Elander P, Linvall M, Lindström B (2005) MiMi—Performance Assessment: Main Report. MiMi Report 2003:3. In: Höglund LO, Herbert RB (eds) MiMi Print. Luleå, Stockholm
INAP (2009) International network for acid prevention. Global Acid Rock Drainage (GARD) Guide™. http://www.gardguide.com/index.php/Main_Page. Accessed 7 June 2011
Koerner RM, Daniel DE (1997) Final covers for solid waste landfills and abandoned dumps. ASCE Press, Reston
Lottermoser B (2010) Mine wastes, characterization. Treatment and environmental impacts. Springer, Berlin
Lundgren T, Lindahl L (1993) Gruvavfall från sulfidmalmsbrytning. SEPA Report 4202 (In Swedish)
Mácsik J, Rogbeck Y, Svedberg B, Uhlander O, Mossakowska A (2003) Fly ash and sewage sludge as liner material—preparation for a pilot test with fly-ash stabilized sewage sludge as landfill liner. Report 837. (In Swedish)
Markewitz K, Cabral AR, Panarotto CT, Lefebvre G (2004) Anaerobic biodegradation of an organic by-products leachate by interaction with different mine tailings. J Hazard Mater 110:93–104
Marklund S (1997) Dewatering of wastewater sludge by natural air drying. Licentiate Dissertation, Luleå University of Technology
Nason PA, Alakangas L, Öhlander B (2010) the effectiveness of using sewage sludge as a sealing layer on sulphide-rich mine tailings: a pilot-scale experiment, northern Sweden. In: International Mine Water Association Symposium 2010: Mine Water and Innovative Thinking, Sydney, N.S. Canada, pp 155–158
Neuschutz C (2009) Phytostabilization of mine tailings covered with fly ash and sewage sludge. Doctoral Dissertation, Stockholm University
Neuschütz C, Isaksson K, Lundmark L, Gregor M (2009) Evaluation of a dry-cover treatment consisting of vegetation, sewage sludge and fly ash. In: Securing the Future: 8th International Conference on Acid Rock Drainage, Skellefteå, Sweden
Nickel E (1973) Experimental dissolution of light and heavy minerals in comparison with weathering and intrastitial solution. Contrib Sedimentol 1:1–68
Peppas A, Komnitsas K, Halikia I (2000) Use of organic covers for acid mine drainage control. Miner Eng 13:563–574
Ritchie AIM (1994) Rates of mechanisms that govern pollutant generation from pyritic wastes. In: American Chemical Society Symposium Series, Washington, DC
Shcherbakova E (2006) Geochemical and hydrological aspects of interactions between water and mine waste. Licentiate Dissertation, Luleå University of Technology
Stehouwer R, Day RL, Macneal KE (2006) Nutrient and trace element leaching following mine reclamation with biosolids. J Environ Qual 35:1118–1126
Wennman P (2004) Decomposition and nitrogen transformations in digested sewage sludge applied to mine tailings-effects of temperature, soil moisture, pH and plants. Licentiate Dissertation, Swedish University of Agricultural Sciences, Uppsala
Acknowledgments
This study was funded by Georange, which distributes EU-structural funds in Sweden, and was supported by the Centre of Advanced Mining and Metallurgy at Luleå University of Technology. The authors wish to express further gratitude to: New Boliden AB for providing access to the Georange Environmental Test Site; Bert-Sive of Bergteamet for field assistance and support at the Kristineberg Mine site; Lucile Villain for field assistance; ALS Scandinavia for providing the sample analyses. Finally thanks goes to Milan Vnuk for help preparing the figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nason, P., Alakangas, L. & Öhlander, B. Using sewage sludge as a sealing layer to remediate sulphidic mine tailings: a pilot-scale experiment, northern Sweden. Environ Earth Sci 70, 3093–3105 (2013). https://doi.org/10.1007/s12665-013-2369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2369-0