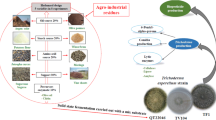
Abstract
Purpose
Fifteen fungal strains were compared with regards to their ability to produce endoglucanase and xylanase from food waste by solid-state fermentation (SSF).
Methods
The fungi were isolated from six different types of composts and they were identified based on rDNA internal transcribed spacer sequence data. The Congo red test was performed for the preliminary screening of fungi for endoglucanase and xylanase production. After the initial screening, the fungi that showed endoglucanase and xylanase producing ability were further tested on the enzymatic activities in food waste through solid-state fermentation. The effects of different parameters including moisture content, incubation temperature, inoculum level, and incubation period on endoglucanase and xylanase production were also evaluated.
Results
Preliminary results indicated that all the fungi, except Absidia sp., had endoglucanase and xylanase production activities. During SSF process, Aspergillus niger showed the highest level of extracellular endoglucanase and xylanase activities, which is 17.37 ± 3.76 and 189.24 ± 2.96 U/g ds, respectively. Moreover, treatment with the strain at normal moisture content (77.67%), 0.5 mL inoculum level at 25 °C incubation temperature for 6 days were the most efficient conditions for endoglucanase and xylanase production (28.81 ± 0.67 and 213.47 ± 10.66 U/g ds, respectively).
Conclusion
This study demonstrated that strain A. niger can be used potentially for enzyme production and proposes a new and economical method to produce high value enzymes with food waste by SSF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food waste means the discard or loss of foodstuff, which mainly contained unsold food, food processing leftovers and uneaten food from residences and commercial establishments such as restaurants and supermarkets [1]. Food waste is produced in every process of the food supply chains. Gooch et al. [2] reported the percentage of waste created throughout the food supply chain in Canada and showed that the waste created at home have the highest percentage (51%). Organic materials account for approximately 40% of the municipal solid waste in Canada [3]. In Canada, more than $30 billion of food waste is thrown out every year and almost four-fifth of that food waste is perfectly edible [2]. Similarly, the single largest composition of municipal solid waste is food wasted in the US [4]. Only in retail and consumer levels, Americans threw away approximately $161.6 billion dollars of food in 2010 [5]. With the rapidly increasing urban populations and economic growth combined with a swiftly expanding catering industry [6], alternative food waste disposal technologies have become a major concern in recent years. Food waste is a global environmental challenge for the waste management, as it has high moisture content (around 80%) and is difficult to handle. Currently, landfilling with other municipal solid wastes is the predominant method for food waste disposal in North America. However, this approach is facing more and more regulations and environmental pressures.
Solid-state fermentation (SSF) provides an extraordinary way to separate organic waste from municipal solid wastes and thereby reducing the amount of waste sent to the landfills. SSF is a technology that produces biomolecules from microorganisms in solid substrates without free-flowing liquid [7]. The nutrient-rich organic waste could be potentially used as a substrate in SSF for generating value-added biomolecules, such as enzymes [8, 9]. Lignocellulolytic enzymes such as endoglucanase and xylanase have many applications in various industries. Endoglucanase is an enzyme that can hydrolyze cellulose. It can be used for industrial food processing, such as in coffee, textile industry, laundry detergents, pulp and paper industry, and also in the pharmaceutical industry. Xylanase was used in animal feed initially and then more recently applied in food, textile, as well as in the paper industries. Tsai et al. [10] reported that it is feasible to use xylanase as feed additives for wheat-based diets or feed that requires high arabinoxylan.
Endoglucanase and xylanase are produced by fungi, bacteria, and actinomycetes, but the most common producers are fungi. The high cost of endoglucanase is mainly due to the substrates used in production, and also the slow growth rate of fungi. According to Polizeli et al. [11], cellulase, xylanase, and pectinase contributed towards one-fifth of the world enzyme market and most of endoglucanase and xylanase are produced through submerged culture. However, SSF has some advantages over the submerged fermentation, e.g. low energy consumption, high production rate, high production yield, and simpler extraction process [12, 13]. Therefore, there is an increasing interest in using SSF to produce endoglucanase and xylanase.
Endoglucanase and xylanase have been produced from agro-industrial substrates, such as olive pomace, wheat bran, oil palm trunk, rice husk, yellow mombin residue, mango residue, and corncob residue [14,15,16,17,18,19,20]; however, no research study has attempted their production using mixed food waste as the substrate. Therefore, the objectives of this study were to evaluate the potential of locally isolated fungi strains for the production of extracellular endoglucanase and xylanase, investigate the feasibility of using mixed food waste as the substrate to produce endoglucanase and xylanase during SSF, and optimize the parameters that influence the maximization of endoglucanase and xylanase production.
Materials and Methods
Strain Isolation and Inoculum Preparation
A total of 15 fungal strains were isolated from six different types of composts (including various combinations of plant debris, worm castings, and wood chips) based on methods described by Ottow [21] and Malloch [22]. Fungal strains were identified in part based on the morphological observations and by the utilization of rDNA internal transcribed spacer sequence data [23]. The fungi were kept in potato dextrose agar (39 g/L PDA) plates and maintained by periodic transfer to fresh media. They were stored at 4 °C until further experiments. In order to prepare inoculum, sporulating PDA plates were flooded with 10 mL of sterile distilled water and spores were collected by dislodging with inoculation needle. The spore suspensions with appropriate dilutions (1 × 107 spores/mL) were used as the inoculum.
Fungi Identification
Nucleic acid extraction protocols and amplification protocols for the fungal rDNA internal transcribed spacer (ITS) regions have been previously described in Hausner et al. [24] and Hausner and Wang [25], respectively. Whole cell DNA was used as the template for amplifying DNA fragments of interest using the OneTaq® DNA polymerase system (New England Biolabs, MA, USA). Primers SSUZ and LSU4 [26] were used to amplify the ITS regions. The PCR primer sequences, amplification conditions, sizes of the expected PCR products, and preparation of sequencing templates for fragments have been previously described [25]. Amplicons were prepared for sequencing with the aid of the Promega Wizard SV Gel and PCR clean-up system (Promega, Madison, WI). Purified PCR products were sequenced in both directions using cycle-sequencing protocols and automated Fluorescent DNA sequence analysis (The Manitoba Institute of Cell Biology DNA sequencing facility, Winnipeg, MB). The NCBI resources such as BLASTn combined with alignment and distance tree options were utilized to find matches for rDNA sequences obtained from strains analysed in this study.
Food Waste Preparation and Characterization
The substrate was a simulated food waste that was obtained based on the typical United States diet as compiled from the USDA [27]. A predetermined amount of food waste was crushed by a blender. The resulting mixed paste (particle size was around \(0.2\,\times\,0.2\,{\text{cm}}\)) was used as the substrate in SSF process. Total solids (TS), volatile solids (VS), and total Kjeldahl nitrogen (TKN) were analyzed using standard methods described by the American Public Health Association [28]. The carbon content of the food waste was determined by the percentage of volatile solids divided by 1.83 [29]. The oven-drying method was used for determination of the moisture content of the food waste. One g of food waste (dry weight) was mixed with 5 mL of distilled water and the suspension was shaken at 180 rpm at room temperature for 20 min. The pH of the mixture’s supernatant was taken as the pH of the food waste. All measurements were conducted in triplicate. Table 1 shows the characteristics of the food waste together with standard deviations. TS and VS values are based on wet weight while total carbon and TKN are based on dry weight.
Preliminary Screening of Endoglucanase and Xylanase Production by Congo Red Test
The Congo red test served as a preliminary screen for endoglucanase and xylanase production. More specifically, the fungi were inoculated at the center of the agar plates, which contained Mandel Weber salts medium and used carboxymethylcellulose (CMC) and xylan as the source of carbon for endoglucanase and xylanase producers, respectively [14]. The agar plates were incubated for 5 days at 25 °C to allow the fungi to secrete enzyme(s). After the incubation period, the agar plates were stained for 15 min with 10 mL aqueous solution of Congo red (0.1% w/v). The Congo red solution was then discarded and the plates were further rinsed by 10 mL of 1 M NaCl. The clear hydrolysis zone around the fungal colony indicated the production of endoglucanase and xylanase by the fungus. For each fungal strain, two replicates of the Congo red test were performed.
Solid-State Fermentation
SSF process was conducted in the 500 mL Erlenmeyer flask and each flask contained 20 g of food waste (wet weight). The wet substrate in the flask was autoclaved at 121 °C for 40 min. Then 1.0 mL spore suspension (1 × 107 spores/mL) of the fungus was inoculated to the substrate until the substrate cooled down to the room temperature. Subsequently, the flasks were incubated for 6 days at 25 °C. The SSF process for every strain was conducted in triplicate.
Optimization of Endoglucanase and Xylanase Production
The production of endoglucanase and xylanase was optimized by following ‘one-variable-at-a-time’ approach to study the effects of different factors on the growth of Aspergillus niger. The effect of various factors including incubation temperature (20, 25, 30, 35, and 40 °C), incubation period (2, 4, 6, 8, and 10 days), initial moisture content (40, 50, 60, 70, and 80%), and inoculum level (0.5, 1.0, 1.5, 2.0, and 2.5 mL) were examined individually.
Crude Endoglucanase and Xylanase Extraction and Analytical Methods
The crude enzymes were extracted as described by Tian and Yuan [30], mixing the fermented matter with 25 mL distilled water containing 2% CaCl2·2H2O on the rotary shaker and shaking at 200 rpm for 1 h at room temperature (22 °C). The mixture was then centrifuged at 1482×g for 0.5 h at 4 °C. The supernatant was used for enzyme analysis.
The activity of endoglucanase and xylanase was determined based on a standard procedure that measures the amount of reducing sugars released by crude enzymes through the dinitrosalicylic acid method as described by Miller [31]. In brief, endoglucanase was determined by incubating 0.9 mL 2% (w/v) CMC in sodium acetate buffer (50 mM, pH 4.7) with 0.1 mL suitably diluted crude enzyme at 50 °C for 40 min. Xylanase activity was determined under the same condition as endoglucanase; however, 2% (w/v) birchwood xylan was used as the reaction substrate. After incubation, the reaction was interrupted by DNS solution (1%). Then the tubes were placed in boiling water for 10 min and subsequently cooled for 5 min in the ice bath. The absorbance was measured spectrophotometrically (BioTek® Instruments, Winooski, USA) at 540 nm. The production of the reducing sugar was deduced from glucose and xylose standard curves. The standard curves were prepared by 5, 10, 15, 20 and 30 mmol/L of the glucose and xylose in acetate buffer, respectively. One unit of enzyme activity was defined as the amount of enzyme required to release 1 µmol of glucose reducing sugar equivalents per minute from 2% (w/v) CMC under 50 °C and pH 4.7. The activity of endoglucanase and xylanase activity was expressed in U per gram of dry substrate.
Statistical Analysis
SPSS (Version 22.0, Armonk, NY, IBM Corp.) was used for statistical analysis and one-way ANOVA was applied for determining the significance of different conditions for the enzyme production. For the multiple comparisons, S–N–K test was used. Treatments were reported to have a significant influence on the result when the P value was < 0.05 (95% confidence level).
Results and Discussion
Fungi Identification
Fungi originally isolated from compost materials were identified with the aid of molecular sequences derived from the rDNA ITS region. The ITS region was amplified and sequenced from all the isolates. Various primer combinations were applied to increase the chances of obtaining ITS amplicons (Table 2). The ITS sequences were used as queries in BLASTn to find similar or identical sequences in the database. Based on sequences extracted from GenBank, isolates were assigned to genera and in some cases to species level (Table 2). The collection of 15 strains based on cultural and molecular variations could be assigned to a range of different families belonging to either the Ascomycota (Genera: Aspergillus, Penicillium, Podospora, Fusarium, Aureobasidium, Trichoderma) or the Zygomycota (Genera: Absidia, Lichtheimia, Umbelopsis). In many instances, Genus level designation was possible but species designations in some cases were challenging; for example, different species of Trichoderma or Penicillium share identical ITS regions. This has been previously noted by Visagie et al. [23], Yilmaz et al. [32] and Jaklitsch [33].
Preliminary Screening of Fungi for Enzyme Production by Congo Red Test
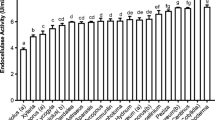
The preliminary screening was performed to observe the growth of 15 fungal strains on agar plates that contained CMC or xylan as the carbon source. The fungi that produced visible clear zones due to hydrolysis in CMC or xylan plates indicate endoglucanase or xylanase activity, respectively. In addition, the bigger the ratio of the hydrolysis zone diameter to the colony diameter, the higher the expected endoglucanase or xylanase activity of the fungus. Among the 15 fungal isolates, only one strain (Absidia sp.) did not show endoglucanase and xylanase activity after 5 days of incubation. Therefore, all the fungi except Absidia sp. were selected for SSF (Table 3).
Screening for Endoglucanase and Xylanase Producers by Solid-State Fermentation
The production of endoglucanase and xylanase in SSF is gaining interest since it is a cost-effective technology with low substrate cost and high enzyme yields [15, 34]. SSF was conducted to assess the enzyme productivity of the fungi that were selected from the preliminary screening and to test the feasibility of using food waste as the substrate for producing endoglucanase and xylanase. The endoglucanase and xylanase production from different fungal strains are presented in Table 4. The strain A. niger showed the highest amount of extracellular endoglucanase and xylanase activities, which were 17.37 ± 3.76 and 189.24 ± 2.96 U/g ds, respectively. The enzymatic activities in some strains were undetectable, which is probably because the enzyme activities produced from these strains were below the detection limit. Moreover, it was observed that xylanase activity was 10.9-fold higher than endoglucanase activity. The reason is that the structure of xylan is considered “weak” and the side chain is easily to be removed [16]. Therefore, xylan can be easily hydrolyzed and xylanase activity is higher than endoglucanase activity. Bansal et al. [15] showed that individual kitchen waste such as carrot peelings, orange peelings, pineapple peelings, and potato peelings pre-treated with H2SO4 and NaOH can be used as the substrate for endoglucanase production in SSF. In this SSF study, the results demonstrated that the strain A. niger was the most efficient endoglucanase and xylanase producer on food waste. Therefore, A. niger was selected for further optimization in SSF.
Optimization of Endoglucanase and Xylanase Production in Solid-State Fermentation
Initial moisture content of the food waste was adjusted to 40, 50, 60, 70, and 80% with the oven-drying method [29] or moistened with distilled water. Endoglucanase and xylanase activities obtained at different moisture content are shown in Table 1 and they are significantly (P < 0.05) affected by moisture content of the food waste. Generally speaking, the enzyme productivity increased dramatically as the moisture content increased. The activities of the endoglucanase were undetectable when the initial moisture content of the food waste was 50% and 60%. Similar endoglucanase activities (10.98 ± 3.63, 11.46 ± 3.52, and 11.33 ± 3.63 U/g ds) were observed at the other three moisture content treatments (40, 70, and 80%, respectively). For xylanase activities, the moisture content of 70 and 80% showed 160.94 ± 8.29 and 159.89 ± 7.62 U/g ds, respectively (Table 1). As compared with high initial moisture content (70 and 80%), xylanase activities (6.76 ± 2.53, 3.43 ± 1.99 and 13.69 ± 7.65 U/g ds) decreased significantly when the initial moisture content was low (40, 50, and 60%). This reduction was mostly likely due to the decrease in the solubility of nutrients in the substrate [9, 35], which consequently affected the growth of the microbial agent and led to the poor production of the enzyme. Besides, moisture content can also interfere with the decomposition rate of the organic matter in the substrate [36] and therefore affect the enzymes productivity in SSF. The endoglucanase and xylanase activities observed from the moisture content of 70 and 80% were lower than the enzyme activities obtained at the normal moisture content in the previous step (17.37 ± 3.76 and 189.24 ± 2.96 U/g ds for endoglucanase and xylanase, respectively). This indicated that the normal moisture content (77.67%) of the food waste was the optimum condition for the enzyme production.
Incubation temperature is another important factor in SSF as it ultimately influences the growth of fungus, the formation and germination of the spore, and metabolic activities such as enzyme production [9]. The results for the effect of incubation temperature on endoglucanase and xylanase activity are shown in Fig. 1. Maximum endoglucanase production occurred at 30 °C with a yield of 13.86 ± 1.03 U/g ds, while endoglucanase yield at 20 °C was not detectable (Fig. 1a). According to the results of the multiple comparisons (S–N–K test), there were no significant differences for endoglucanase production at 25, 30, 35, and 40 °C. In addition, low temperature means lower energy requirement in the SSF process, which is more economical. Besides, water evaporation at high temperature is faster than it in the low temperature. Therefore, 25 °C was the economical choice in subsequent experiments for endoglucanase production. For xylanase production, the activities (206.23 ± 4.95 U/g ds) obtained at 25 °C was significantly (P < 0.05) higher than the xylanase yield at other temperatures. The xylanase productivity decreased when the incubation temperature increased and the lowest xylanase activity (10.79 ± 4.09 U/g ds) was observed at 40 °C. Bansal et al. [15] reported that high temperatures can alter the constituent of the cell membrane, stimulate protein catabolism, and induce cell death. Therefore, 25 °C was again selected as the incubation temperature for further experiments.
Effect of incubation temperature A. niger on the a endoglucanase production and b xylanase production; Letters shared in common between or among the groups indicate no significant difference according to S–N–K test at the significance level of 0.05; error bars are standard deviations of three replicates
The inoculated flasks were incubated for different time periods ranging from 2 to 10 days. Endoglucanase and xylanase activities in the substrate were tested at every second day. Endoglucanase and xylanase activities were significantly affected by fermentation period (Fig. 2). The results showed that SSF with 6 and 10 days incubation significantly increased endoglucanase production. The maximum endoglucanase activity occurred after 10-day incubation with the endoglucanase yield of 36.07 ± 12.43 U/g ds and increases 3.64-fold as compared with 2-day incubation (9.92 ± 2.07 U/g ds) (Fig. 2a). However, the endoglucanase production obtained at 6 and 10 days does not have a significant difference (P > 0.05), which indicated that longer fermentation time does not result in a significant improvement in endoglucanase production. Similarly, longer incubation periods resulted in poor xylanase activities (Fig. 2b) and 6 days incubation period showed the maximum amount of xylanase production (213.47 ± 10.66 U/g ds). For longer incubation periods, the xylanase production decreased at 8 and 10 days, which were significantly (P < 0.05) lower than the xylanase yield at 6 days of incubation. The observation that enzyme yield decreased at longer incubation periods is in agreement with findings of Bansal et al. [15] and dos Santos et al. [37]. Besides, Mrudula and Murugammal [35] reported that endoglucanase activity increased steadily at the beginning but longer incubation period reduced the enzyme production after it reached maximum activity at 72 h of incubation. In general, a longer incubation period can result in a decrease in enzyme production due to the reduction of nutrients in the substrate, the release of proteases, and the drop in the pH in the substrate [15, 30].
Conclusions
This study highlighted a strain isolated from compost, A. niger, which could produce high-levels of endoglucanase and xylanase from food waste by SSF. Maximum endoglucanase and xylanase production (28.81 ± 0.67 and 213.47 ± 10.66 U/g ds, respectively) were achieved using normal moisture content (77.67%) at 25 °C for 6 days incubation. Therefore, food waste from municipal solid waste can be used as a potential substrate for endoglucanase and xylanase production. This technique could reduce the enzyme production cost by lowering the substrate cost in the enzyme industry and potentially alleviate environmental issues caused by food waste.
References
Fisgativa, H., Tremier, A., Dabert, P.: Characterizing the variability of food waste quality: a need for efficient valorisation through anaerobic digestion. Waste Manag. 50, 264–274 (2016)
Gooch, M., Felfel, A., Marenick, N.: Food Waste in Canada: Opportunities to Increase the Competitiveness of Canada’s Agri-food Sector, While Simultaneously Improving the Environment. Value Chain Management Center, Guelph (2010)
Environment Canada: Technical document on municipal solid waste organics processing. Canada. https://www.ec.gc.ca/gdd-mw/3E8CF6C7-F214-4BA2-A1A3-163978EE9D6E/13-047-ID-458-PDF_accessible_ANG_R2-reduced%20size.pdf (2013). Accessed 10 June 2017
US EPA: Municipal solid waste in the United States: 2007 facts and figures. Office of Solid Waste and Emergency Response, Washington, DC. http://large.stanford.edu/publications/coal/references/docs/msw07-rpt.pdf (2008). Accessed 21 April 2017
Jean, C.B., Hodan, F.W., Jeffrey, H.: The estimated amount, value, and calories of postharvest food losses at the retail and consumer levels in the United States. United States Department of Agriculture. https://www.ers.usda.gov/webdocs/publications/eib121/43680_eib121.pdf (2014). Accessed 14 June 2017
Li, Y., Jin, Y.: Effects of thermal pretreatment on acidification phase during two-phase batch anaerobic digestion of kitchen waste. Renew. Energy 77, 550–557 (2015)
Pandey, A.: Recent process developments in solid-state fermentation. Process Biochem. 27(2), 109–117 (1992)
Pandey, A., Soccol, C.R., Mitchell, D.: New developments in solid state fermentation: I-bioprocess-products. Process Biochem. 35(10), 1153–1169 (2000)
Pandey, A.: Solid-state fermentation. Biochem. Eng. J. 13(2), 81–84 (2003)
Tsai, T., Dove, C.R., Cline, P.M., Owusu-Asiedu, A., Walsh, M.C., Azain, M.: The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livestock Sci. 197, 46–52 (2017)
Polizeli, M.L.T.M., Rizzatti, A.C.S., Monti, R., Terenzi, H.F., Jorge, J.A., Amorim, D.S.: Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67(5), 577–591 (2005)
Chen, H.: Modern Solid State Fermentation, Theory and Practice. Springer, Heidelberg (2013)
Pandey, A., Selvakumar, P., Soccol, C.R., Nigam, P.: Solid-state fermentation for the production of industrial enzymes. Curr. Sci. 77, 149–152 (1999)
Leite, P., Salgado, J.M., Venâncio, A., Domínguez, J.M., Belo, I.: Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour. Technol. 214, 737–746 (2016)
Bansal, N., Tewari, R., Soni, S.K.: Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manag. 32(7), 1341–1346 (2012)
Ang, S.K., Shaza, E.M., Adibah, Y., Suraini, A.A., Madihah, M.S.: Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 48(9), 1293–1302 (2013)
Xia, L., Cen, P.: Cellulase production by solid state fermentation on lignocellulosic waste from the xylose industry. Process Biochem. 34(9), 909–912 (1999)
Marques, G.L., dos Santos Reis, N., Silva, T.P., Ferreira, M.L.O., Aguiar-Oliveira, E., de Oliveira, J.R., Franco, M.: Production and characterisation of xylanase and endoglucanases produced by Penicillium roqueforti ATCC 10110 through the solid-state fermentation of rice husk residue. Waste Biomass Valor. (2017). https://doi.org/10.1007/s12649-017-9994-x
de Almeida Antunes Ferraz, J.L., Souza, L.O., Soares, G.A., Coutinho, J.P., de Oliveira, J.R., Aguiar-Oliveira, E., Franco, M.: Enzymatic saccharification of lignocellulosic residues using cellulolytic enzyme extract produced by Penicillium roqueforti ATCC 10110 cultivated on residue of yellow mombin fruit. Bioresour. Technol. (2017). https://doi.org/10.1016/j.biortech.2017.06.048
dos Santos, T.C., Cavalcanti, I.S., Bonomo, R.C.F., Santana, N.B., Franco, M.: Optimisation of productions of cellulolytic enzymes by Aspergillus niger using residue of mango a substrate. Ciencia Rural (2011). https://doi.org/10.1590/S0103-84782011005000145
Ottow, J.C.G.: Rose bengal as a selective aid in the isolation of fungi and actinomycetes from natural sources. Mycologia 64, 304–315 (1972)
Malloch, D.: Moulds, Their Isolation, Cultivation and Identification. University of Toronto Press, Toronto (1981)
Visagie, C.M., Houbraken, J., Frisvad, J.C., Hong, S.B., Klaassen, C.H.W., Perrone, G., Seifert, K.A., Varga, J., Yaguchi, T., Samson, R.A.: Identification and nomenclature of the genus Penicillium. Stud. Mycol. 78, 343–371 (2014)
Hausner, G., Eyjólfsdóttir, G.G., Reid, J., Klassen, G.R.: Two additional species of the genus Togninia. Can. J. Bot. 70, 724–734 (1992)
Hausner, G., Wang, X.: Unusual compact rDNA gene arrangements within some members of the Ascomycota: evidence for molecular co-evolution between ITS1 and ITS2. Genome 48, 648–660 (2005)
Hausner, G., Iranpour, M., Kim, J.-J., Breuil, C., Davis, C.N., Gibb, E.A., Reid, J., Loewen, P.C., Hopkin, A.A.: Fungi vectored by the introduced bark beetle Tomicus piniperda in Ontario, Canada, and comments on the taxonomy of Leptographium lundbergii, Leptographium terebrantis, Leptographium truncatum, and Leptographium wingfieldii. Can. J. Bot. 83, 1222–1237 (2005)
Kim, M., Chowdhury, M.M.I., Nakhla, G., Keleman, M.: Characterization of typical household food wastes from disposers: fractionation of constituents and implications for resource recovery at wastewater treatment. Bioresour. Technol. 183, 61–69 (2015)
APHA: Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC (2005)
Barrington, S., Choinière, D., Trigui, M., Knight, W.: SE-Structures and environment: compost airflow resistance. Biosyst. Eng. 81(4), 433–441 (2002)
Tian, M., Yuan, Q.: Optimization of phytase production from potato waste using Aspergillus ficuum. 3 Biotech (2016). https://doi.org/10.1007/s13205-016-0573-9
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959)
Yilmaz, N., Visagie, C.M., Houbraken, J., Frisvad, J.C., Samson, R.A.: Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 78, 175–341 (2014)
Jaklitsch, W.M.: European species of Hypocrea Part I. The green-spored species. Stud. Mycol. 63, 1–91 (2009)
Singhania, R.R., Sukumaran, R.K., Pillai, A., Prema, P., Szakacs, G., Pandey, A.: Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J. Biotechnol. 5, 332–336 (2006)
Mrudula, S., Murugammal, R.: Production of cellulase by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz. J. Microbiol. 42(3), 1119–1127 (2011)
Gautam, P., Sabu, A., Pandey, A., Szakacs, G., Soccol, C.R.: Microbial production of extra-cellular phytase using polystyrene as inert solid support. Bioresour. Technol. 83(3), 229–233 (2002)
dos Santos, T.C., Gomes, D.P.P., Bonomo, R.C.F., Franco, M.: Optimisation of solid state fermentation of potato peel for the production of celluloytic enzymes. Food Chem. 133(4), 1299–1304 (2012)
Acknowledgements
This work was financially supported by Green Manitoba (WRAPP 14–028) and Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-05510).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Tian, M., Wai, A., Guha, T.K. et al. Production of Endoglucanase and Xylanase Using Food Waste by Solid-State Fermentation. Waste Biomass Valor 9, 2391–2398 (2018). https://doi.org/10.1007/s12649-017-0192-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0192-7