Abstract
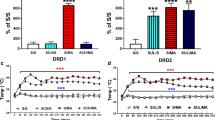
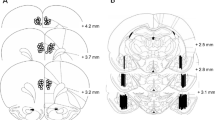
The nucleus accumbens (Nacc) and medial prefrontal cortex (mPFC) receive dopaminergic innervation from the ventral tegmental area and are involved in learning. Male rats with 6-hydroxydopamine (6-OHDA)-induced dopaminergic and noradrenergic reductions in the Nacc or mPFC were tested for allocentric and egocentric learning to determine their role in these forms of neuroplasticity. mPFC dopaminergic and noradrenergic reductions did not result in changes to either type of learning or memory. Nacc dopaminergic and noradrenergic reductions resulted in allocentric learning and memory deficits in the Morris water maze (MWM) on acquisition, reversal, and probe trials. MWM cued performance was also affected, but straight-channel swim times and swim speed during hidden platform trials in the MWM were not affected. Nacc dopaminergic and noradrenergic reductions also impaired egocentric learning in the Cincinnati water maze (CWM). Nacc-lesioned animals tested in the CWM in an alternate path through the maze were not significantly affected. 6-OHDA injections in the Nacc resulted in 63 % dopamine and 62 % norepinephrine reductions in the Nacc and 23 % reductions in adjacent dorsal striatum. 6-OHDA injections in the mPFC resulted in 88 % reductions in dopamine and 59 % reductions in norepinephrine. Hence, Nacc dopamine and/or norepinephrine play a role in egocentric and allocentric learning and memory, while mPFC dopamine and norepinephrine do not.






Similar content being viewed by others
References
Abercrombie ED, Zigmond MJ (1989) Partial injury to central noradrenergic neurons: reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. J Neurosci 9:4062–4067
Aguirre GK, D’Esposito M (1999) Topographical disorientation: a synthesis and taxonomy. Brain 122(Pt 9):1613–1628
Annett LE, McGregor A, Robbins TW (1989) The effects of ibotenic acid lesions of the nucleuc accumbens on spatial learning and extinction in the rat. Behav Brain Res 31:231–242
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202
Braun AA, Graham DL, Schaefer TL, Vorhees CV, Williams MT (2012) Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol Learn Mem 97:402–408
Braun AA, Amos-Kroohs RM, Gutierrez A, Lundgren KH, Seroogy KB, Skelton MR, Vorhees CV, Williams MT (2015) Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiol Learn Mem 118:55–63
Bubser M (1994) 6-Hydroxydopamine lesions of the medial prefrontal cortex of rats do not affect dopamine metabolism in the basal ganglia at short and long postsurgical intervals. Neurochem Res 19:421–425
Bubser M, Schmidt WJ (1990) 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res 37:157–168
Butters N, Soeldner C, Fedio P (1972) Comparison of parietal and frontal lobe spatial deficits in man: extrapersonal vs personal (egocentric) space. Percept Mot Skills 34:27–34
Byrne RW (1982) Geographical knowledge and orientation. Normality and pathology in cognitive functions. Academic Press, London, pp 239–264
Castaneda E, Whishaw IQ, Robinson TE (1990) Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci 10:1847–1854
Coccurello R, Adriani W, Oliverio A, Mele A (2000) Effect of intra-accumbens dopamine receptor agents on reactivity to spatial and non-spatial changes in mice. Psychopharmacology 152:189–199
Commins S, Cunningham L, Harvey D, Walsh D (2003) Massed but not spaced training impairs spatial memory. Behav Brain Res 139:215–223
Cools AR, Ellenbroek B, Heeren D, Lubbers L (1993) Use of high and low responders to novelty in rat studies on the role of the ventral striatum in radial maze performance: effects of intra-accumbens injections of sulpiride. Can J Physiol Pharmacol 71:335–342
Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS (2003) Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol Learn Mem 79:236–242
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
de Bruin JP, Sanchez-Santed F, Heinsbroek RP, Donker A, Postmes P (1994) A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res 652:323–333
de Bruin JP, Swinkels WA, de Brabander JM (1997) Response learning of rats in a Morris water maze: involvement of the medical prefrontal cortex. Behav Brain Res 85:47–55
de Bruin JP, Moita MP, de Brabander HM, Joosten RN (2001) Place and response learning of rats in a Morris water maze: differential effects of fimbria fornix and medial prefrontal cortex lesions. Neurobiol Learn Mem 75:164–178
de Leonibus E, Pascucci T, Lopez S, Oliverio A, Amalric M, Mele A (2007) Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology 194:517–525
Ethier K, Le MN, Rompre PP, Godbout R (2001) Spatial strategy elaboration in egocentric and allocentric tasks following medial prefrontal cortex lesions in the rat. Brain Cogn 46:134–135
Ferretti V, Florian C, Costantini VJ, Roullet P, Rinaldi A, de Leonibus E, Oliverio A, Mele A (2005) Co-activation of glutamate and dopamine receptors within the nucleus accumbens is required for spatial memory consolidation in mice. Psychopharmacology 179:108–116
Floresco SB (2007) Dopaminergic regulation of limbic-striatal interplay. J Psychiatry Neurosci 32:400–411
Floresco SB (2013) Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci 7:62
Garber PA (2000) Evidence for the use of spatial, temporal, and social information by some primate foragers. In: Boinski SB, Garber PA (eds) On the move: How and why animals travel in groups. University of Chicago Press, Chicago, pp 261–298
Gonzalez-Burgos I, Fletes-Vargas G, Gonzalez-Tapia D, Gonzalez-Ramirez MM, Rivera-Cervantes MC, Martinez-Degollado M (2012) Prefrontal serotonin depletion impairs egocentric, but not allocentric working memory in rats. Neurosci Res 73:321–327
Goto Y, Grace AA (2008) Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31:552–558
Grigoryan G, Hodges H, Mitchell S, Sinden JD, Gray JA (1996) 6-OHDA lesions of the nucleus accumbens accentuate memory deficits in animals with lesions to the forebrain cholinergic projection system: effects of nicotine administration on learning and memory in the water maze. Neurobiol Learn Mem 65:135–153
Hagan JJ, Alpert JE, Morris RGM, Iversen SD (1983) The effects of central catecholamine depletions on spatial learning in rats. Behav Brain Res 9:83–104
Haluk DM, Floresco SB (2009) Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology 34:2041–2052
Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT (2008) Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology 199:637–650
Kesner RP, Farnsworth G, DiMattia BV (1989) Double dissociation of egocentric and allocentric space following medial prefrontal and parietal cortex lesions in the rat. Behav Neurosci 103:956–961
King VR, Corwin JV (1992) Spatial deficits and hemispheric asymmetries in the rat following unilateral and bilateral lesions of posterior parietal or medial agranular cortex. Behav Brain Res 50:53–68
Kolb B, Whishaw IQ (1983) Dissociation of the contributions of the prefrontal, motor, and parietal cortex to the control of movement in the rat: an experimental review. Can J Psychol 37:211–232
Kolb B, Pittman K, Sutherland RJ, Whishaw IQ (1982) Dissociation of the contributions of the prefrontal cortex and dorsomedial thalamic nucleus to spatially guided behavior in the rat. Behav Brain Res 6:365–378
Kolb B, Buhrmann K, McDonald R, Sutherland RJ (1994) Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb Cortex 4:664–680
Koob GF (1992) Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13:177–184
Lacroix L, White I, Feldon J (2002) Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res 133:69–81
Lindner MD, Cain CK, Plone MA, Frydel BR, Blaney TJ, Emerich DF, Hoane MR (1999) Incomplete nigrostriatal dopaminergic cell loss and partial reductions in striatal dopamine produce akinesia, rigidity, tremor and cognitive deficits in middle-aged rats. Behav Brain Res 102:1–16
Ma YY, Tian BP, Wilson FA (2003) Dissociation of egocentric and allocentric spatial processing in prefrontal cortex. NeuroReport 14:1737–1741
Ma YY, Ryou JW, Kim BH, Wilson FA (2004) Spatially directed movement and neuronal activity in freely moving monkey. Prog Brain Res 143:513–520
Ma Y, Hu X, Wilson FA (2012) The egocentric spatial reference frame used in dorsal-lateral prefrontal working memory in primates. Neurosci Biobehav Rev 36:26–33
Maaswinkel H, Gispen WH, Spruijt BM (1996) Effects of an electrolytic lesion of the prelimbic area on anxiety-related and cognitive tasks in the rat. Behav Brain Res 79:51–59
McDonald RJ, White NM (1994) Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol 61:260–270
Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C (2002) Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull 58:41–47
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Mogilnicka E (1986) Increase in beta- and alpha 1-adrenoceptor binding sites in the rat brain and in the alpha 1-adrenoceptor functional sensitivity after the DSP-4-induced noradrenergic denervation. Pharmacol Biochem Behav 25:743–746
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Mura A, Feldon J (2003) Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Mov Disord 18:860–871
Nelson AJ, Thur KE, Marsden CA, Cassaday HJ (2010) Dissociable roles of dopamine within the core and medial shell of the nucleus accumbens in memory for objects and place. Behav Neurosci 124:789–799
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Ploeger GE, Spruijt BM, Cools AR (1994) Spatial localization in the Morris water maze in rats: acquisition is affected by intra-accumbens injections of the dopaminergic antagonist haloperidol. Behav Neurosci 108:927–934
Pohl W (1973) Dissociation of spatial discrimination deficits following frontal and parietal lesions in monkeys. J Comp Physiol Psychol 82:227–239
Poucet B (1989) Object exploration, habituation, and response to a spatial change in rats following septal or medial frontal cortical damage. Behav Neurosci 103:1009–1016
Poucet B (1990) A further characterization of the spatial problem-solving deficit induced by lesions of the medial frontal cortex in the rat. Behav Brain Res 41:229–237
Ragozzino ME, Kesner RP (2001) The role of rat dorsomedial prefrontal cortex in working memory for egocentric responses. Neurosci Lett 308:145–148
Rawson T, O’Kane M, Talk A (2010) The medial prefrontal cortex and memory of cue location in the rat. Neurobiol Learn Mem 93:132–136
Redish AD, Touretzky DS (1997) Cognitive maps beyond the hippocampus. Hippocampus 7:15–35
Rich EL, Shapiro M (2009) Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci 29:7208–7219
Robinson TE, Casteneda E, Whishaw IQ (1990) Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can J Psychol 44:253–275
Sargolini F, Florian C, Oliverio A, Mele A, Roullet P (2003) Differential involvement of NMDA and AMPA receptors within the nucleus accumbens in consolidation of information necessary for place navigation and guidance strategy of mice. Learn Mem 10:285–292
Selden NR, Cole BJ, Everitt BJ, Robbins TW (1990) Damage to ceruleo-cortical noradrenergic projections impairs locally cued but enhances spatially cued water maze acquisition. Behav Brain Res 39:29–51
Semmes J, Weinstein S, Ghent L, Teuber HL (1963) Correlates of impaired orientation in personal and extrapersonal space. Brain 86:747–772
Setlow B, McGaugh JL (1998) Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav Neurosci 112:603–610
Smith-Roe SL, Sadeghian K, Kelley AE (1999) Spatial learning and performance in the radial arm maze is impaired after N-methyl-D-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci 113:703–717
Sutherland RJ, Rodriguez AJ (1989) The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behav Brain Res 32:265–277
Sutherland RJ, Kolb B, Whishaw IQ (1982) Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett 31:271–276
Taghzouti K, Louilot A, Herman JP, Le MM, Simon H (1985) Alternation behavior, spatial discrimination, and reversal disturbances following 6-hydroxydopamine lesions in the nucleus accumbens of the rat. Behav Neural Biol 44:354–363
Tirado-Santiago G, Lazaro-Munoz G, Rodriguez-Gonzalez V, Maldonado-Vlaar CS (2006) Microinfusions of neurotensin antagonist SR 48692 within the nucleus accumbens core impair spatial learning in rats. Behav Neurosci 120:1093–1102
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50
Tzschentke TM (2001) Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 63:241–320
U’Prichard DC, Reisine TD, Yamamura S, Mason ST, Fibiger HC, Ehlert F, Yamamura HI (1980) Differential supersensitivity of beta-receptor subtypes in rat cortex and cerebellum after central noradrenergic denervation. Life Sci 26:355–364
Velazquez-Zamora DA, Gonzalez-Ramirez MM, Beas-Zarate C, Gonzalez-Burgos I (2011) Egocentric working memory impairment and dendritic spine plastic changes in prefrontal neurons after NMDA receptor blockade in rats. Brain Res 1402:101–108
Vorhees CV (1987) Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol 9:235–241
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Vorhees CV, Williams MT (2014) Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol Teratol 45:75–90
Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR (1991) An analysis of factors influencing complex water maze learning in rats: effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol 13:213–222
Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT (2008) Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci 26:599–610
Whishaw IQ, Dunnett SB (1985) Dopamine depletion, stimulation or blockade in the rat disrupts spatial navigation and locomotion dependent upon beacon or distal cues. Behav Brain Res 18:11–29
Williams MT, Herring NR, Schaefer TL, Skelton MR, Campbell NG, Lipton JW, McCrea AE, Vorhees CV (2007) Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘foxy’) to adult rats: a new drug of abuse. Neuropsychopharmacology 32:1404–1420
Acknowledgments
This research was supported by NIH project Grants ES015689, MH101609, and training Grant ES007051 (A. A. B., R. M. A-K., A. G.) and the Gardner Family Center for Parkinson’s Disease and Movement Disorders.
Author contributions
This work was part of Dr. Amanda Braun’s doctoral dissertation project. She did the surgical procedures, injected 6-OHDA, oversaw postoperative care, tested the rats in the maze procedures, performed the statistical analyses of the data, created the figures, and drafted the manuscript. Dr. Robyn Amos-Kroohs performed the HPLC monoamine assays. Graduate student Arnold Gutierrez euthanized the rats, dissected and froze the brains for later HPLC, and perfused other brains for histological verification of injection sites. Dr. Kim Seroogy was a collaborator for histology and his research assistant, Kirsten Lundgren, performed the histology. Dr. Williams is Dr. Braun’s graduate research advisor; he played an integral role in the design, interpretation, and writing of the manuscript as well as of her entire dissertation series of experiments. Dr. Vorhees was chair of Dr. Braun’s dissertation committee, is a close collaborator of Dr. Williams, and was involved in the planning, design, and interpretation of the experiments and in the writing/editing of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest concerning this research.
Rights and permissions
About this article
Cite this article
Braun, A.A., Amos-Kroohs, R.M., Gutierrez, A. et al. 6-Hydroxydopamine-Induced Dopamine Reductions in the Nucleus Accumbens, but not the Medial Prefrontal Cortex, Impair Cincinnati Water Maze Egocentric and Morris Water Maze Allocentric Navigation in Male Sprague–Dawley Rats. Neurotox Res 30, 199–212 (2016). https://doi.org/10.1007/s12640-016-9616-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9616-6