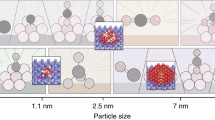
Abstract
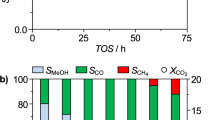
The catalytic performance is highly related to the catalyst structure. Herein, a series of Ni nanoparticles supported on Y2O3 with different morphologies were successfully synthesized via hydrothermal process screening different pH environments. These Ni/Y2O3 catalysts were applied to efficiently produce COx-free H2 through ammonia decomposition. We identify a significant impact of Y2O3 supports on nickel nanoclusters sizes and dispersion. The experimental results show that Ni/Y11 catalyst achieves 100% ammonia decomposition conversion under a gas hour space velocity (GHSV) of 12,000 ml·h−1·gcat−1 and temperature of 650 °C. Such a high level of activity over Ni/Y11 catalyst was attributed to a large specific surface area, appropriate alkalinity, and small Ni nanoparticles diameter with high dispersion.
Graphical abstract

摘要
催化性能与催化剂结构密切相关,本工作通过调控水热法溶液的pH值合成了一系列不同形貌的Y2O3载体,采用浸渍法制备了系列Ni/Y2O3催化剂及其应用于氨分解生成不含碳氧化合物的氢气。研究表明Y2O3 载体对镍纳米颗粒的尺寸和分散度具有显著的影响:Ni/Y11催化剂具有最小的Ni纳米颗粒和较高的Ni分散度,在空速为12,000 ml h−1 gcat−1和温度为650 ºC时实现了氨的完全分解。 Ni/Y11催化剂的高活性主要归因于较大的比表面积、适宜的表面酸碱性和高分散度的Ni纳米颗粒。








Similar content being viewed by others
References
Lu WQ, Zhang RJ, Sam T, Xu R, Zhou FY, Sun Z, Sun ZQ. Microchannel structure design for hydrogen supply from methanol steam reforming. Chem Eng J. 2022;429:132286. https://doi.org/10.1016/j.cej.2021.132286.
Basile F, Benito P, Fornasari G, Vaccari A. Hydrotalcite-type precursors of active catalysts for hydrogen production. Appl Clay Sci. 2010;48(1–2):250. https://doi.org/10.1016/j.clay.2009.11.027.
Mukherjee S, Devaguptapu SV, Sviripa A, Lund CR, Wu G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl Catal B. 2018;226:162. https://doi.org/10.1016/j.apcatb.2017.12.039.
Zhang ZC, Liu GG, Cui XY, Gong Y, Yi D, Zhang QH, Zhu CZ, Saleem F, Chen B, Lai ZC, Yun QB, Cheng HF, Huang ZQ, Peng Y, Fan Z, Li B, Dai W, Chen W, Du Y, Ma L, Sun CJ, Hwang I, Chen S, Song L, Ding F, Gu L, Zhu Y, Zhang H. Evoking ordered vacancies in metallic nanostructures toward a vacated Barlow packing for high-performance hydrogen evolution. Sci Adv. 2021;7(13):eaab6647. https://doi.org/10.1126/sciadv.abd6647.
Holladay JD, Hu J, King DL, Wang Y. An overview of hydrogen production technologies. Catal Today. 2009;139(4):244. https://doi.org/10.1016/j.cattod.2008.08.039.
Guo HX, Wang BY, Shen LH. Fe, Mn based nitrogen carrier in the chemical looping ammonia synthesis technology. Acta Petrolei Sinica. 2020;6(36):1354. https://doi.org/10.3969/j.issn.1001-8719.2020.06.026.
Jin Y, Zhang Z, Yang H, Wang PT, Shen CQ, Cheng T, Huang XQ, Shao Q. Boosting hydrogen production with ultralow working voltage by selenium vacancy-enhanced ultrafine platinum–nickel nanowires. SmartMat. 2022;3(1):130. https://doi.org/10.1002/smm2.1083.
Jing H, Zhu P, Zheng X, Zhang Z, Wang D, Li Y. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv Powder Mater. 2022;1(1):100013. https://doi.org/10.1016/j.apmate.2021.10.004.
Chen Z, Wu K, Huang HW, Cao CF, Yu L, Chen CQ, Lin L, Chaktong Au, Jiang LL. Spatial confinement of electron-rich Ni nanoparticles for efficient ammonia decomposition to hydrogen production. ACS Catal. 2021;11(6):10345. https://doi.org/10.1021/acscatal.1c02420.
Schüth F, Palkovits R, Schlögl R, Su DS. Ammonia as a possible element in an energy infrastructure: catalysts for ammonia decomposition. Energy Environ Sci. 2012;5(4):6278. https://doi.org/10.1039/c2ee02865d.
Li L, Shi L, Yu XH, Qing SJ, Gao ZX, Luo QQ, Feng G, Zhang RB. Adsorption of Nin (n = 1–4) clusters on perfect and O-defective CuAl2O4 surfaces: a DFT study. Chinese Chem Lett. 2019;30(6):1147. https://doi.org/10.1016/j.cclet.2019.03.047.
Yin S, Xu B, Zhou X, Au CT. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl Catal A. 2004;277(1–2):1. https://doi.org/10.1016/j.apcata.2004.09.020.
Wei C, Liu ZE, Li CF, Singh S, Lu HR, Gong YD, Li PP, Wang HL, Yang X, Xu M, Mu SJ. Status of an MWth integrated gasification fuel cell power-generation system in China. Int J Coal Sci Technol. 2021;8(3):401. https://doi.org/10.1007/s40789-021-00429-1.
Wang HQ, Zhang WJ, Zhang XW, Hu SX, Zhang ZC, Zhou WJ, Liu H. Multi-interface collaboration of graphene cross-linked NiS-NiS2-Ni3S4 polymorph foam towards robust hydrogen evolution in alkaline electrolyte. Nano Res. 2021;14(12):4857. https://doi.org/10.1007/s12274-021-3445-5.
Qin Y, Zhang W, Wang F, Li J, Ye J, Sheng X, Li C, Liang X, Liu P, Wang X, Zheng X, Ren Y, Xu C, Zhang Z. Extraordinary p–d hybridization interaction in heterostructural Pd-PdSe nanosheets boosts C−C bond cleavage of ethylene glycol electrooxidation. Angew Chem Int Ed. 2022;61(16):e202200899. https://doi.org/10.1002/anie.202200899.
Qin YC, Wang FQ, Wang XM, Wang MW, Zhang WL, An WK, Wang XP, Ren YL, Zheng X, Lv DC, Ahmad A. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion. Rare Met. 2021;40(9):2354. https://doi.org/10.1007/s12598-021-01727-y.
Wang F, Zhang W, Wan H, Li C, An W, Sheng X, Liang X, Wang X, Ren Y, Zheng X, Lv D, Qin Y. Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction. Chinese Chem Lett. 2022;33(5):2259. https://doi.org/10.1016/j.cclet.2021.08.074.
Yin S. Nano Ru/CNTs: a highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Appl Catal B. 2004;48(4):237. https://doi.org/10.1016/j.apcatb.2003.10.013.
Yin SF, Zhang QH, Xu BQ, Zhu WX, Ng CF, Au CT. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J Catal. 2004;224(2):384. https://doi.org/10.1016/j.jcat.2004.03.008.
Choudhary TV, Sivadinarayana C, Goodman DW. Catalytic ammonia decomposition: COx-free hydrogen production for fuel cell applications. Catal Lett. 2001;72(3):197. https://doi.org/10.1023/A:1009023825549.
Ganley JC, Thomas FS, Seebauer EG, Masel RI. A priori catalytic activity correlations: the difficult dase of hydrogen production from ammonia. Catal Lett. 2004;96(3–4):117. https://doi.org/10.1023/B:CATL.0000030108.50691.d4.
Hu ZP, Weng CC, Chen C, Yuan ZY. Two-dimensional mica nanosheets supported Fe nanoparticles for NH3 decomposition to hydrogen. Mol Catal. 2018;448:162. https://doi.org/10.1016/j.mcat.2018.01.038.
Su Q, Gu LL, Yao Y, Zhao J, Ji WJ, Ding WP, Au CT. Layered double hydroxides derived Nix(MgyAlzOn) catalysts: enhanced ammonia decomposition by hydrogen spillover effect. Appl Catal B. 2017;201:451. https://doi.org/10.1016/j.apcatb.2016.08.051.
Varisli D, Kaykac NG. COx free hydrogen production over cobalt incorporated silicate structured mesoporous catalysts. Appl Catal B. 2012;127:389. https://doi.org/10.1016/j.apcatb.2012.08.042.
Hajduk Š, Dasireddy VD, Likozar B, Dražić G, Orel ZC. COx-free hydrogen production via decomposition of ammonia over Cu-Zn-based heterogeneous catalysts and their activity/stability. Appl Catal B. 2017;211:57. https://doi.org/10.1016/j.apcatb.2017.04.031.
Gong XY, Gu YQ, Li N, Zhao HY, Jia CJ, Du YP. Thermally stable hierarchical nanostructures of ultrathin MoS2 nanosheet-coated CeO2 hollow spheres as catalyst for ammonia decomposition. Inorg Chem. 2016;55(8):3992. https://doi.org/10.1021/acs.inorgchem.6b00265.
Muroyama H, Saburi C, Matsui T, Eguchi K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl Catal A. 2012;443–444:119. https://doi.org/10.1016/j.apcata.2012.07.031.
Huang CQ, Yu YZ, Yang JM, Yan Y, Wang DS, Hu FY, Wang XW, Zhang RB, Feng G. Ru/La2O3 catalyst for ammonia decomposition to hydrogen. Appl Surf Sci. 2019;476:928. https://doi.org/10.1016/j.apsusc.2019.01.112.
Okura K, Okanishi T, Muroyama H, Matsui T, Eguchi K. Promotion effect of rare-earth elements on the catalytic decomposition of ammonia over Ni/Al2O3 catalyst. Appl Catal A. 2015;505:77. https://doi.org/10.1016/j.apcata.2015.07.020.
Yao LH, Li YX, Zhao J, Ji WJ, Au CT. Core–shell structured nanoparticles (M@SiO2, Al2O3, MgO; M=Fe Co, Ni, Ru) and their application in COx-free H2 production via NH3 decomposition. Catal Today. 2010;158(3–4):401. https://doi.org/10.1016/j.cattod.2010.05.009.
Ju XH, Liu L, Yu P, Guo JP, Zhang XL, He T, Wu GT, Chen P. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Appl Catal B. 2017;211:167. https://doi.org/10.1016/j.apcatb.2017.04.043.
Nakamura I, Fujitani T. Role of metal oxide supports in NH3 decomposition over Ni catalysts. Appl Catal A. 2016;524:45. https://doi.org/10.1016/j.apcata.2016.05.020.
Durak-Çetin Y, Sarıoğlan Ş, Sarıoğlan A, Okutan H. The effect of support type on the activity of zeolite supported iron catalysts for the decomposition of ammonia. React Kinet Mech Cat. 2016;118(2):683. https://doi.org/10.1007/s11144-016-0981-1.
Raróg-Pilecka W, Miśkiewicz E, Jodzis S, Petryk J, Łomot D, Kaszkur Z, Karpiński Z, Kowalczyk Z. Carbon-supported ruthenium catalysts for NH3 synthesis doped with caesium nitrate: activation process, working state of Cs–Ru/C. J Catal. 2006;239(2):313. https://doi.org/10.1016/j.jcat.2006.01.035.
Li C, He J, Ma Y. Sintering behavior and thermal conductivity of Y2O3 fully stabilized HfO2 ceramics. Rare Met. 2021;40(5):1255. https://doi.org/10.1007/s12598-020-01421-5.
Zhao JB, Wu LL. Yb3+-and Er3+-doped Y2O3 microcrystals for upconversion photoluminescence and energy transfer with enhancements of near-ultraviolet emission. Rare Met. 2021;40(1):123. https://doi.org/10.1007/s12598-019-01269-4.
Feng J, Liu L, Ju XH, Wang JM, Zhang XL, He T, Chen P. Highly dispersed ruthenium nanoparticles on Y2O3 as superior catalyst for ammonia decomposition. ChemCatChem. 2021;13(6):1552. https://doi.org/10.1002/cctc.202001930.
Okura K, Okanishi T, Muroyama H, Matsui T, Eguchi K. Ammonia decomposition over nickel catalysts supported on rare-earth oxides for the on-site generation of hydrogen. ChemCatChem. 2016;8(18):2988. https://doi.org/10.1002/cctc.201600610.
Huang CQ, Li HX, Yang JM, Wang CQ, Hu FY, Wang XW, Lu ZH, Feng G, Zhang RB. Ce0.6Zr0.3Y0.1O2 solid solutions-supported Ni-Co bimetal nanocatalysts for NH3 decomposition. Appl Surf Sci. 2019;478:708. https://doi.org/10.1016/j.apsusc.2019.01.269.
Yu YZ, Gan YM, Huang CQ, Lu ZH, Wang XW, Zhang RB, Feng G. Ni/La2O3 and Ni/MgO-La2O3 catalysts for the decomposition of NH3 into hydrogen. Int J Hydrog Energy. 2020;45(33):16528. https://doi.org/10.1016/j.ijhydene.2020.04.127.
Dupont A, Parent C, Le Garrec B, Heintz JM. Size and morphology control of Y2O3 nanopowders via a sol-gel route. J Solid State Chem. 2003;171(1–2):152. https://doi.org/10.1016/s0022-4596(02)00202-5.
Wang X, Li Y. Rare-earth-compound nanowires, nanotubes, and fullerene-like nanoparticles: synthesis, characterization, and properties. Chem Eur J. 2003;9(22):5627. https://doi.org/10.1002/chem.200304785.
Sing K. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem. 1985;57:603. https://doi.org/10.1351/pac198557040603.
Bellido JD, Assaf EM. Effect of the Y2O3-ZrO2 support composition on nickel catalyst evaluated in dry reforming of methane. Appl Catal A. 2009;352(1):179. https://doi.org/10.1016/j.apcata.2008.10.002.
Yan Y, Dai Y, Yang Y, Lapkin AA. Improved stability of Y2O3 supported Ni catalysts for CO2 methanation by precursor-determined metal-support interaction. Appl Catal B. 2018;237:504. https://doi.org/10.1016/j.apcatb.2018.06.021.
Italiano C, Llorca J, Pino L, Ferraro M, Antonucci V, Vita A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl Catal B. 2020;264:118494. https://doi.org/10.1016/j.apcatb.2019.118494.
Liu J, Li CM, Wang F, He S, Chen H, Zhao YF, Wei M, Evans DG, Duan X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal Sci Technol. 2013;3(10):2627. https://doi.org/10.1039/c3cy00355h.
Liu B, Li C, Zhang G, Yao X, Chuang SS, Li Z. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods. ACS Catal. 2018;8(11):10446. https://doi.org/10.1021/acscatal.8b00415.
Jia X, Zhang X, Rui N, Hu X, Liu CJ. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl Catal B. 2019;244:159. https://doi.org/10.1016/j.apcatb.2018.11.024.
Rui N, Wang ZY, Sun KH, Ye JY, Ge QF, Liu CJ. CO2 hydrogenation to methanol over Pd/In2O3: effects of Pd and oxygen vacancy. Appl Catal B. 2017;218:488. https://doi.org/10.1016/j.apcatb.2017.06.069.
Bu K, Kuboon S, Deng J, Li H, Yan T, Chen G, Shi L, Zhang D. Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts. Appl Catal B. 2019;252:86. https://doi.org/10.1016/j.apcatb.2019.04.007.
Dębek R, Radlik M, Motak M, Galvez ME, Turek W, Da Costa P, Grzybek T. Ni-containing Ce-promoted hydrotalcite derived materials as catalysts for methane reforming with carbon dioxide at low temperature–On the effect of basicity. Catal Today. 2015;257:59. https://doi.org/10.1016/j.cattod.2015.03.017.
Pavel OD, Tichit D, Marcu IC. Acido-basic and catalytic properties of transition-metal containing Mg–Al hydrotalcites and their corresponding mixed oxides. Appl Clay Sci. 2012;61:52. https://doi.org/10.1016/j.clay.2012.03.006.
Li YY, Men Y, Liu S, Wang JG, Wang K, Tang YH, An W, Pan XL, Li L. Remarkably efficient and stable Ni/Y2O3 catalysts for CO2 methanation: effect of citric acid addition. Appl Catal B. 2021;293:120206. https://doi.org/10.1016/j.apcatb.2021.120206.
Hongmanorom P, Ashok J, Chirawatkul P, Kawi S. Interfacial synergistic catalysis over Ni nanoparticles encapsulated in mesoporous ceria for CO2 methanation. Appl Catal B. 2021;297:120454. https://doi.org/10.1016/j.apcatb.2021.120454.
Liu P, Derchi M, Hensen EJ. Promotional effect of transition metal doping on the basicity and activity of calcined hydrotalcite catalysts for glycerol carbonate synthesis. Appl Catal B. 2014;144:135. https://doi.org/10.1016/j.apcatb.2013.07.010.
Zhao L, Li XY, Qu ZP, Zhao QD, Liu SM, Hu XJ. The NiAl mixed oxides: the relation between basicity and SO2 removal capacity. Sep Purif Technol. 2011;80(2):345. https://doi.org/10.1016/j.seppur.2011.04.035.
Tejuca LG, Bell AT, Fierro JL, Peña MA. Surface behaviour of reduced LaCoO3 as studied by TPD of CO, CO2 and H2 probes and by XPS. Appl Surf Sci. 1988;31(3):301. https://doi.org/10.1016/0169-4332(88)90095-5.
Jurado L, Papaefthimiou V, Thomas S, Roger AC. Upgrading syngas from wood gasification through steam reforming of tars over highly active Ni-perovskite catalysts at relatively low temperature. Appl Catal B. 2021;299:120687. https://doi.org/10.1016/j.apcatb.2021.120687.
Ni J, Leng W, Mao J, Wang J, Lin J, Jiang D, Li X. Tuning electron density of metal nickel by support defects in Ni/ZrO2 for selective hydrogenation of fatty acids to alkanes and alcohols. Appl Catal B. 2019;253:170. https://doi.org/10.1016/j.apcatb.2019.04.043.
Zhang PZ, Han F, Yan JY, Qiao XL, Guan QX, Li W. N-doped ordered mesoporous carbon (N-OMC) confined Fe3O4-FeCx heterojunction for efficient conversion of CO2 to light olefins. Appl Catal B. 2021;299 120639. https://doi.org/10.1016/j.apcatb.2021.120639.
Ma HB, Zhang RB, Huang SF, Chen WQ, Shi QJ. Ni/Y2O3-Al2O3 catalysts for hydrogen production from steam reforming of ethanol at low temperature. J Rare Earth. 2012;30(7):683. https://doi.org/10.1016/s1002-0721(12)60112-4.
Jesus JC, Gonzalez I, Quevedo A, Puerta T. Thermal decomposition of nickel acetate tetrahydrate: an integrated study by TGA, QMS and XPS techniques. J Mol Catal A. 2005;228(1–2):283. https://doi.org/10.1016/j.molcata.2004.09.065.
Elu SV, Suzuki K, Vijayaraj M, Barman S, Gopinath CS. In situ XPS investigations of Cu1−xNixZnAl-mixed metal oxide catalysts used in the oxidative steam reforming of bio-ethanol. Appl Catal B. 2005;55(4):287. https://doi.org/10.1016/j.apcatb.2004.09.007.
Wang N, Qian WZ, Chu W, Wei F. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming. Catal Sci Technol. 2016;6(10):3594. https://doi.org/10.1039/c5cy01790d.
Requies J, Cabrero MA, Barrio VL, Guemez MB, Cambra JF, Arias PL, Perez-Alonso FJ, Ojeda M, Pena MA, Fierro JL. Partial oxidation of methane to syngas over Ni/MgO and Ni/La2O3 catalysts. Appl Catal A. 2005;289(2):214. https://doi.org/10.1016/j.apcata.2005.05.002.
Huang X, Xue GX, Wang CZ, Zhao N, Sun NN, Wei W, Sun YH. Highly stable mesoporous NiO-Y2O3-Al2O3 catalysts for CO2 reforming of methane: effect of Ni embedding and Y2O3 promotion. Catal Sci Technol. 2016;6(2):449. https://doi.org/10.1039/c5cy01171j.
Wang YH, Liu HM, Xu BQ. Durable Ni/MgO catalysts for CO2 reforming of methane: activity and metal–support interaction. J Mol Catal A. 2009;299(1–2):44. https://doi.org/10.1016/j.molcata.2008.09.025.
Kugai J, Subramani V, Song C, Engelhard MH, Chin YH. Effects of nanocrystalline CeO2 supports on the properties and performance of Ni–Rh bimetallic catalyst for oxidative steam reforming of ethanol. J Catal. 2006;238(2):430. https://doi.org/10.1016/j.jcat.2006.01.001.
Duan XZ, Qian G, Liu Y, Ji J, Zhou XG, Chen D, Yuan WK. Structure sensitivity of ammonia decomposition over Ni catalysts: a computational and experimental study. Fuel Process Technol. 2013;108:112. https://doi.org/10.1016/j.fuproc.2012.05.030.
Sato K, Abe N, Kawagoe T, Miyahara SI, Honda K, Nagaoka K. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. Int J Hydrog Energy. 2017;42(10):6610. https://doi.org/10.1016/j.ijhydene.2016.11.150.
Astsumi R, Noda R, Takagi H, Vecchione L, Andrea DC, Zaccaria DP, Kuramoto K. Ammonia decomposition activity over Ni/SiO2 catalysts with different pore diameters. Int J Hydrog Energy. 2014;39(26):13954. https://doi.org/10.1016/j.ijhydene.2014.07.003.
Sima D, Wu H, Tian K, Xie S, Foo JJ, Li S, Wang D, Ye Y, Zheng Z, Liu Y. Enhanced low temperature catalytic activity of Ni/Al-Ce0.8Zr0.2O2 for hydrogen production from ammonia decomposition. Int J Hydrog Energy. 2020;45(16):9342. https://doi.org/10.1016/j.ijhydene.2020.01.209.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Nos. 21868016, 21763018, 22005296 and 21875096), the Key Laboratory for Environment and Energy Catalysis of Jiangxi Province (No. 20181BCD40004), the Natural Science Foundation of Jiangxi Province (No. 20181BAB203016) and the Graduate Students Innovation Special Foundation of Jiangxi Province (No. YC2021-B014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, RB., Tu, ZA., Meng, S. et al. Engineering morphologies of yttrium oxide supported nickel catalysts for hydrogen production. Rare Met. 42, 176–188 (2023). https://doi.org/10.1007/s12598-022-02136-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02136-5