Abstract
Commercial A356 alloy was refined with a homemade Al–5Ti–0.25C–2RE master alloy, and the microstructure and macrostructure of the refined alloy were investigated. The results show that the grain refining effect of A356 is poor by the addition level of 0.5 wt% master alloy, but when the level reaches 3.0 wt% the grain can get a satisfactory refining effect. Dendrite of A356 can be effectively refined by addition of 0.5 wt% master alloy; however, the refining effect is not significantly improved by further increasing the addition of master alloy. Grain and dendrite refining effects are compared in this article, and the results show that the grain and dendrite exhibit different refining effects with the same addition level of master alloy. Dendrite is easier to reach the optimal refining effect than grain.
Graphical Abstract
Although 0.5 wt% master alloy can effectively refine SDAS, it has a weak effect on grain size. By increasing the addition level, the grain can get a better refining effect, but the SDAS refinement has no obvious improvement.

Similar content being viewed by others
1 Introduction
Grain size and secondary dendrite arm spacing (SDAS) are two main standards for evaluating the refining effect of aluminum and its alloy. Grain refinement can not only improve the mechanical properties of alloys but also reduce its sensitivity of shearing stress and time of homogenization effectively and bring a series of excellent qualities to aluminum alloy [1, 2]. As an important parameter in Al–Si alloys, SDAS has a close relationship with alloy’s mechanical properties, microsegregation, and the distribution of shrinkage porosity and many other microdefects [3, 4]. Therefore, fining dendrite structure and reducing the SDAS have a practical significance in improving the properties of alloys.
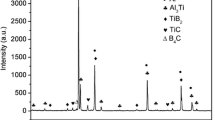
A356 alloys are most popular in the aeronautic and automotive industries. However, coarse grains and dendrite in A356 lead to low mechanism properties. Al–Ti–B master alloys are most commonly used in commercial A356, whereas TiB2 particles are easily aggregated in the melt and get poisoned by Zr, Cr, and Mn [5, 6]. Al–Ti–C was found to be free from the problems of Al–Ti–B, but the poor wettability between graphite and liquid aluminum made the fabrication and application of Al–Ti–C grain refiners become very difficult in industry [7]. In our previous experiments, a certain amount of RE was added in preparing Al–Ti–C, and the poor wettability was successfully solved [8].
Some researchers stressed that the grain size was the weighting standard in evaluating the refining effect of Al–Si alloys [7, 9], and others claimed SDAS was the criterion [10–12], but the grain refinement and dendrite refinement were not considered simultaneously. In this article, commercial A356 alloy was refined with a homemade Al–5Ti–0.25C–2RE master alloy, and the microstructure and macrostructure of refined A356 alloys were observed and analyzed.
Grain refinement and dendrite refinement were evaluated, respectively, and the refining mechanism were also discussed.
2 Experimental
First, the commercial A356 alloy was placed into a graphite crucible and melted at 760 °C in a qualified resistance furnace. After the A356 alloy was fully melted, the temperature was decreased to 720 °C, and then a certain amount of Al–5Ti–0.25C–2RE master alloy was added into the melted A356; after holding for 3 min, the melt was stirred slightly for 30 s to make sure the master alloy was dispersed uniformly into the melt. After holding for a certain time, the melt was poured into a cast iron mold at 720 °C. The bar has a dimension of Φ25 mm × 100 mm. Samples were cut at 45 mm from the bottom of the bar. Macrospecimens were etched with a mixed acid of 60 % HCl + 30 % HNO3 + 5 % HF + 5 % H2O. Microspecimens were grinded, polished, and etched with a reagent of 0.5 vol% HF, and then the microstructure was analyzed by optical microscopy. An image analysis software (Image J) was used in measuring and calculating the grain size and SDAS. The Brinell hardness (BH) of A356 alloys was tested by BH-A5 Brinell microhardness tester.
3 Results and discussion
3.1 Grain refinement of A356 by Al–5Ti–0.25C–2RE
Figure 1 is the macrostructures of A356 with different addition levels of Al–5Ti–0.25C–2RE. Figure (1a) shows the largest equiaxial grains distributed homogeneously in A356 commercial alloy. When adding the master alloy into the A356, grain size decreases. A356 shows a poor refining effect when the adding quantity of the master alloy is 0.5 or 1.0 wt%, as shown in Fig. 1(b) and (c). Increasing the addition level up until to 3.0 wt%, A356 can get a satisfactory refining effect, as shown in Fig. 1e.
Table 1 shows BH of commercial A356 refined by adding Al–5Ti–0.25C–2RE, which indicates that the BH of A356 increases with decreasing grain size. The BH of A356 refined by 3.0 wt% Al–5Ti–0.25C–2RE increased by 16.9 % compared with the initial commercial A356 alloy.
3.2 Dendrite refinement of A356 by Al–5Ti–0.25C–2RE
Figure 2 shows the microstructure of A356 refined by Al–5Ti–0.25C–2RE. As shown in Fig. 2(a), cast A356 alloy without grain refiner shows long strip and coarse dendrite grains. When adding 3.0 wt% Al–5Ti–0.25C–2RE master alloy into A356, the significant refinement of dendrite can be seen from Fig. 2(b). Long acicular eutectic silicon is replaced by short and pointillize silicon.
Figure 3 shows the refining effect of A356 with different addition levels of Al–5Ti–0.25C–2RE and different holding periods. SDAS was measured and calculated from Fig. 3, and its developing trend is shown in Fig. 4. From Figs. 3 and 4, we can observe that, with a certain addition level, dendrite exhibits a weak refinement with 5 min holding time. Dendrite can get an optimal refining effect by increasing the holding time to 30 min. When further increasing the holding time to 60 min, refining shows a fading effect. Dendrite of A356 with 0.5 wt% master alloy shows the best refining effect by holding for 30 min. Although dendrite can be further refined by increasing the master alloy, refining effect is not obvious when the addition amount of master is above 3.0 wt%.
3.3 Comparison of grain refinement and dendrite refinement
Figure 5 shows the developing trend of SDAS and grain size. The comparison between grain refinement and dendrite refinement shows that SDAS can be refined from 25.482 to 21.748 μm by addition 0.5 wt% master alloy, and SDAS is reduced by 14.7 %. When the master alloy is increased to 3.0 wt%, SDAS is only reduced by 4.2 % compared with alloy with 0.5 wt% master alloy. Grain size can only be refined from 1,230 to 910 μm by addition of 0.5 wt% master alloy, so the refining effect is very poor. However, by further adding the master alloy to 3.0 wt%, grain size is effectively refined to 230 μm. To sum up, although 0.5 wt% master alloy can effectively refine SDAS, it has a weak effect on grain size. Increasing the addition level, the grain can get a better refining effect, but the SDAS refinement has no obvious improvement.
3.4 Discussion
Through the above analysis, it can be concluded that the grain and dendrite are refined in different ways and the refining mechanism is different.
In the grain refining process, Al–Ti–C master alloy releases TiC particles to the melt. As the crystal structure and lattice parameter of TiC are similar to α-Al, TiC can stimulate α-Al nucleates effectively [13]. Nafisi and Banerji proposed that TiC particles are unstable in the melt in the way that they are easily transformed into Al4C3. As the structure of Al4C3 is hexagonal, and Al4C3 has a poor matching ability with α-Al, which results in TiC loss its nucleation ability [14, 15]. In our previous experiment, the Al–5Ti–0.25C–2RE master alloy was used to refine pure aluminum, and a good refining effect was obtained with only 0.5 wt% master alloy. In the refining process of pure aluminum, TiAl3 and Ti2Al20Ce released Ti atoms to the melt. Ti atoms were easily segregated on the surface of TiC, and a layer rich in Ti was formed. The layer can not only protect TiC from changing into Al4C3, but also promote the nucleation of α-Al by peritectic reaction, and more detail was represented in Ref. [8]. When compared with the optimal addition level for pure aluminum, it is interesting to find that 0.5 wt% master alloy nearly has no effect on A356, as shown in Fig. 1(b). Mohanty and Gruzieski [16] proposed that the high content of Si in A356 is prone to react with Ti and formed a ternary Al–Ti–Si phase, which would lead to the loss of free Ti in the melt and damage the Ti–rich layer. Losing the protection of Ti–rich layer, TiC would change into Al4C3 easily and lose the nucleation precursor. For the newly formed Al–Ti–Si in pure Al, it can react with the melt and promote the nucleation of α-Al. However, because the nucleation temperature is very low [17], the nucleation is ineffective. All the factors referred above result in the weak refining effect of 0.5 wt% master alloy for A356. However, when increasing the addition level of master alloy, the refining effect becomes better, as shown in Fig. 1(e). The responsible reason is that the master alloy introduces much more newly free Ti to the melt. Meanwhile, Ti2Al20Ce releases Ti and Al4Ce to the melt. Al4Ce is also an effective nucleation phase for α-Al, so the refining effect for A356 becomes better with the increasing of master alloy, as shown in Fig. 1.
Casting temperature, cooling rate, alloy composition, and its own properties are the main factors for SDAS in the solidification process. Chen and Kattamis [18] proposed that SDAS can be expressed by
where t f is the local solidification time, \( \Upgamma \) is the Gibbs–Thompson coefficient, D L is the solute diffusion coefficient in the liquid, C L is the liquid phase concentration, C 0 is the original concentration of the liquid, m L is slope of liquid, and k is the equilibrium partition coefficient.
As shown in Eqs. (1) and (2), SDAS is mainly influenced by M and the local solidification time t f. M is affected by alloy composition and diffusion coefficient. When keeping the alloy composition at a certain, λ is mainly affected by D L and some others factors that are related to D L, such as casting temperature and cooling rate. In this experiment, alloys are casted and cooled in the same condition, so M has little influence on SDAS. In the refining process, Ti2Al20Ce releases La and Ce to the melt, and the atomic radiuses of La and Ce are 0.274 and 0.270 nm, respectively. However, Al atomic radius is only 0.182 nm, so La and Ce are easily supplanted to the interdendrite region. The constitutional supercooling by the segregation of rare earth can effectively reduce the local solidification time t f, so little addition of master alloy can effectively refine the SDAS. At the same time, Ti can also make an effort in confining the growth of dendrite [19]. That is the reason why 0.5 wt% master alloy has a weak effect on grain refinement but can effectively refine the dendrite. The poor dendrite refinement by increasing the addition of master alloy is because the segregation of rare earth reached a saturation state. The newly added Ti can promote the refining process, but the effect is very weak. Thus, further increasing master alloy addition has no significant effect for dendrite refining, as shown in Fig. 3.
4 Conclusion
A small amount (0.5 wt%) of Al–5Ti–0.25C–2RE is weak to the grain refining effect, but when increasing the addition level to 3.0 wt%, A356 can get a satisfactory grain refining effect. 0.5 wt% Al–5Ti–0.25C–2RE can effectively refine the SDAS; while further increasing the addition level, there is no significant influence for dendrite refinement. Grain and dendrite are refined in different ways, and dendrite can get the optimal refining effect more easily than grain.
References
Wang X, Chen GQ, Li B, Wu LM, Jiang DM. Effects of Sc, Zr and Ti on the microstructure and properties of Al alloys with high Mg content. Rare Met. 2010;29(1):66.
Zhao HL, Zhou ZX, Liu XD, Guan SK. Influence of Mg3N2 powder on microstructures and mechanical properties of AZ31Mg alloy. J Cent S Univ Technol. 2008;15(4):459.
Easton M, Davidson C, Stjohn D. Effect of alloy composition on the dendrite arm spacing of multicomponent aluminum alloys. Metall Mater Trans A. 2010;41(6):1528.
Li W, Liao HC, Lu B, Xi X. The effect of RE on Sr modifying Al-13 % Si alloy. Foundry. 2009;58(4):364.
Li YL, Feng HK, Cao FR, Cheng YB, Gong LY. Effect of high density ultrasonic on the microstructure and refining property of Al–5Ti–0.25C grain refiner alloy. Metall Mater Trans A. 2008;487(1):518.
Kumar GS, Murty BS, Chakraborty M. Development of Al–Ti–C grain refiners and study of their grain refining efficiency on Al and Al–7Si alloy. J Alloy Compd. 2005;396(1–2):143.
Li DW, Ding YZ, Zhe LW, Li GH, Wei S, Yong LC. The refinement effect of Al–Ti–C–RE master alloy prepared by adding Ce2O3 on pure Al. Adv Mater Res. 2010;139:227.
Zhao H, Song Y, Li M, Guan S. Grain refining efficiency and microstructure of Al–Ti–C–RE master alloy. J Alloy Compd. 2010;508(1):206.
Birol Y. A novel Al–Ti–B alloy for grain refining Al–Si foundry alloys. J Alloy Compd. 2009;486(1–2):219.
Ni H, Sun BD, Jiang HY, Ding WJ. Effect of RE on SDAS of A356 alloy. Chin J Nonferrous Metals. 2002;12(5):940.
Prasada RAK, Das K, Murty BS, Chakraborty M. Microstructural features of as-cast A356 alloy inoculated with Sr, Sb modifiers and Al–Ti–C grain refiner simultaneously. Mater Lett. 2008;62(2):273.
Guan RG, Cao FR, Chen LQ, Li JP, Wang C. Dynamical solidification behaviors and microstructural evolution during vibrating wavelike sloping plate process. J Mater Process Technol. 2009;209(5):2592.
Karantzalis A, Lekatou A, Georgatis E, Tsiligiannis T, Mavros Η. Solidification observations of dendritic cast Al alloys reinforced with TiC particles. J Mater Eng Perform. 2010;19(9):1268.
Nafisi S, Ghomashchi R. Grain refining of conventional and semi-solid A356 Al–Si alloy. J Mater Process Technol. 2006;174(1–3):371.
Banerji A, Reif W. Metallographic investigation of TiC nucleants in the newly developed Al–Ti–C grain refiner. J Mater Sci. 1994;29(7):1958.
Mohanty PS, Gruzieski JE. Grain refinement of aluminium by TiC. Scr Metall Mater. 1994;31(2):179.
Chen XG, Fortier M. TiAlSi intermetallic formation and its impact on the casting processing in Al–Si alloys. J Mater Process Technol. 2010;210(13):1780.
Chen M, Kattamis TZ. Dendrite coarsening during directional solidification of Al–Cu–Mn alloys. Mater Sci Eng A. 1998;239–247:239.
Zhang Z, Hosoda S, Kim IS, Watanabe Y. Grain refining performance for Al and Al–Si alloy casts by addition of equal-channel angular pressed Al-5 mass% Ti alloy. Mater Sci Eng A. 2006;425(1–2):55.
Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (No. 51174177).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhao, HL., Yue, JS., Gao, Y. et al. Grain and dendrite refinement of A356 alloy with Al–Ti–C–RE master alloy. Rare Met. 32, 12–17 (2013). https://doi.org/10.1007/s12598-013-0021-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-013-0021-5