Abstract
Clinical trials are often designed to assess the effect of therapeutic interventions on the incidence of recurrent events in the presence of a dependent terminal event such as death. Statistical methods based on multistate analysis have considerable appeal in this setting since they can incorporate changes in risk with each event occurrence, a dependence between the recurrent event and the terminal event, and event-dependent censoring. To date, however, there has been limited development of statistical methods for the design of trials involving recurrent and terminal events. Based on the asymptotic distribution of regression coefficients from a multiplicative intensity Markov regression model, we derive sample size formulas to address power requirements for both the recurrent and terminal event processes. We consider the design of trials for which separate marginal hypothesis tests are of interest for the recurrent and terminal event processes and deal with both superiority and non-inferiority tests. Simulation studies confirm that the designs satisfy the nominal power requirements in both settings, and an application to a trial evaluating the effect of a bisphosphonate on skeletal complications is given for illustration.


Similar content being viewed by others
References
Andersen PK, Borgan Ø, Gill RD, Keiding N (1993) Statistical models based on counting processes. Springer, New York
George SL, Desu MM (1974) Planning the size and duration of a clinical trial studying the time to some critical event. J Chronic Dis 27:15–24
Freedman LS (1982) Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1:121–129
Schoenfeld DA (1983) Sample-size formula for the proportional-hazards regression model. Biometrics 39:499–503
Lachin JM, Foulkes MA (1986) Evaluation of sample size and power for analyses of survival with allowance for non-uniform patient entry, losses to follow-up, non-compliance and stratification. Biometrics 42:507–519
Cook RJ (1995) The design and analysis of randomized trials with recurrent events. Stat Med 14:2081–2098
Lawless JF, Nadeau JC (1995) Nonparametric estimation of cumulative mean functions for recurrent events. Technometrics 37:158–168
Cook RJ, Lawless JF, Nadeau C (1996) Robust tests for treatment comparisons based on recurrent event responses. Biometrics 52:557–571
Bernardo MVP, Harrington DP (2001) Sample size calculations for the two-sample problem using the multiplicative intensity model. Stat Med 20:557–579
Fleming TR, Harrington DP (1991) Counting processes and survival analysis. Wiley, New York
Wei LJ, Lin DY, Weissfeld L (1989) Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc 84:1065–1073
Hughes MD (1997) Power considerations for clinical trials using multivariate time-to-event data. Stat Med 16:865–882
Song R, Kosork MR, Cai J (2008) Robust covariate-adjusted log-rank statistics and corresponding sample size formula for recurrent events data. Biometrics 64:741–750
Prentice RL, Williams BJ, Peterson AV (1981) On the regression analysis of multivariate failure time data. Biometrika 68:373–379
Rothmann M, Li N, Chen G, Chi GYH, Temple R, Tsou H-H (2003) Design and analysis of non-inferiority mortality trials in oncology. Stat Med 22:239–264
Kaul S, Diamond GA (2006) Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 145:62–69
D’Agostino RB, Massaro JM, Sullivan LM (2003) Non-inferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med 22:169–186
Cook RJ, Lee K-A, Li H (2007) Non-inferiority trial design for recurrent events. Stat Med 26:4563–4577
Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C, Wheeler H, Simeone JF, Seaman JJ, Knight RD, Heffernan M, Mellars K, Reitsma DJ (1998) Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 16:2038–2044
Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD (1996) Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 335:1785–1791
Cook RJ, Lawless JF (2007) The statistical analysis of recurrent events. Springer, New York
Cook RJ, Lawless JF, Lakhal-Chaieb L, Lee K-A (2009) Robust estimation of mean functions and treatment effects for recurrent events under event-dependent censoring and termination: application to skeletal complications in cancer metastatic to bone. J Am Stat Assoc 104:60–75
Andersen PK, Gill RD (1982) Cox regression model for counting processes: a large sample study. Ann Stat 10:1100–1120
Cole EH, Cattran DC, Farewell VT, Aprile M, Bear RA, Pei YP, Fenton SS, Tober JA, Cardella CJ (1994) A comparison of rabbit antithymocyte serum and OKT3 as prophylaxis against renal allograft rejection. Transplantation 57:60–67
Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J (TORCH investigators) (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356:775–789
Hubard RA, Inoue LYT, Fann JR (2008) Modeling nonhomogeneous Markov processes via time transformation. Biometrics 64:843–850
Aalen OO, Borgan Ø, Fekjær H (2001) Covariate adjustment of event histories estimated from Markov chains: the additive approach. Biometrics 57:993–1001
Datta S, Satten GA (2001) Validity of the Aalen-Johansen estimators of stage occupation probabilities and Nelson–Aalen estimators of integrated transition hazards for non-Markov models. Stat Probab Lett 55:403–411
Ghosh D, Lin DY (2000) Nonparametric analysis of recurrent events and death. Biometrics 56:554–562
Boher J, Cook RJ (2006) Implications of model misspecification in robust tests for recurrent events. Lifetime Data Anal 12:69–95
Huang CY, Wang MC (2004) Joint modeling and estimation for recurrent event processes and failure time data. J Am Stat Assoc 99:1153–1165
Liu L, Wolfe RA, Huang X (2004) Shared frailty models for recurrent events and a terminal event. Biometrics 60:747–756
Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, Brouste V, Soubeyran P (2007) Joint frailty models for recurring events and death using maximum penalized likelihood estimation: application on cancer events. Biostatistics 8:708–721
Kalbfleisch JD, Lawless JF (1985) The analysis of panel data under a Markov assumption. J Am Stat Assoc 80:863–871
Gill RD (1980) Censoring and stochastic integrals. Mathematical centre tracts, vol 124. Mathematical Centrum, Amsterdam
Cox DR, Miller HD (1965) The theory of stochastic processes. Wiley, New York
Horn RA, Johnson CR (1994) Topics in matrix analysis. Cambridge University Press, Cambridge
Moler C, Van Loan C (2003) Nineteen dubious ways to compute the exponential of a matrix, twenty-five years later. SIAM Rev 45:3–49
Moler C, Van Loan C (1978) Nineteen dubious ways to compute the exponential of a matrix. SIAM Rev 20:801–836
Higham NJ (2005) The scaling and squaring method for the matrix exponential revisited. SIAM J Matrix Anal Appl 26:1179–1193
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada (LW and RJC) and the Canadian Institutes for Health Research (RJC). Richard Cook is a Canada Research Chair in Statistical Methods for Health Research. The authors thank Jerry Lawless for helpful discussions, the editorial reviewers for helpful comments, and Novartis Pharmaceuticals for permission to use the data from the clinical trial.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Asymptotic Equivalence of the Partial Score Statistics
Under the null hypothesis, \(m^{-\frac{1}{2}}\) times the partial score statistic (6) can be written as

Using similar arguments in the proofs of Theorems 4.2.1 and 4.3.1 of Gill [35], one can show that the second term of the above expression converges in probability to zero as m→∞ for every β 0.
Similarly, let

be the associated martingale process for the recurrent event under the alternative hypothesis. One can write the partial score statistic (6) under the alternative hypothesis as follows:

Using similar arguments as for the null hypothesis, one can show the second term converges in probability to zero as m→∞ for every β 0. We now show the last term of the above expression converges in probability to zero as m→∞. From the regularity conditions of Andersen and Gill [23], the integrand is locally bounded for every u∈(0,τ]. Note that the last term can be written as

where \(R_{j}^{(a)}(\beta_{0}, u)\) converges almost surely to \(E_{A}(R_{j}^{(a)}(\beta_{0}, u))\) at each time point u, a=0,1. It follows from the Slutsky’s theorem that the first term of the integrand in (28) converges almost surely to zero as m→∞ at every u. By the central limit theorem,

converges in distribution to a normal random variable at every u with mean μ as dΛ 0j (u)P(τ i >u) times
and the variance

Then, for every u the integrand in (28) converges in probability to zero. Therefore, it follows from the Lebesgue’s dominated convergence theorem that (28) converges in probability to zero as m→∞.
A similar approached can be used to prove the asymptotic equivalence of the partial score statistics (7) is (14) under the null hypothesis and (16) under the alternative hypothesis.
1.2 Evaluation of Expectations Under the True Model
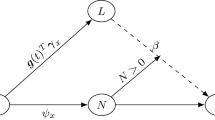
The necessary expectations require the evaluation of the probability being in state j at time t, P(Z i (t)=j|Z i (0)=0), for the proposed Markov process in Fig. 1. As an example, the calculation of \(E_{0}(\bar{Y}_{ij}(u)e^{\beta_{0} v_{i}} d\varLambda_{0j}(u) )\) in (10), is carried out as follows:

The transition probabilities are computed as described in the following section.
1.3 Evaluation of the Transition Probability Matrix
The evaluation of expectations under particular models requires the calculation of the Markov transition probability matrix; for notational convenience we suppress the dependence on i. We consider a finite state space with J+1 states corresponding to the cumulative number of recurrent events from 0 to J and one absorbing state D for the terminal event. For 0≤s≤t, let P(s,t|v) be the (J+2)×(J+2) transition probability matrix with (k,ℓ) entry

for ℓ=k+1 or D, k=0,1,…,J. Let Q v(t) denote the transition intensity matrix for individuals in treatment group v, the elements of which are based on the intensities λ k (t|H(t)) and γ k (t|H(t)) defined in Sect. 2.
For a time-homogeneous process adopted at the design stage, let λ k (t|H(t))=λ k and γ k (t|H(t))=γ k be the intensities for k−1→k and k−1→D transitions, respectively. The transition intensity matrix can then be written simply as Q v. and has (k,ℓ) entry given by λ k for k=1,…,J and ℓ=k+1, γ k for k=1,…,J and ℓ=J+2, −(λ k +γ k ) for k=ℓ=1,…,J, and zero otherwise. Under such a time-homogeneous Markov model, P(s,s+t)=P(0,t)=P(t) and P(t)=exp(Q v t).
There are several approaches available to compute P(t) for a given transition intensity matrix Q v. If Q v has J+2 linearly independent eigenvectors, let A be a matrix of eigenvectors, and note that AQ v A −1 is a diagonal matrix with the eigenvalues d 1,d 2,…,d J+2 of Q v along its diagonal. Then by the spectral value decomposition [34],
If Q v does not have J+2 linearly independent eigenvectors, the Jordan canonical form can be used instead [36]. For some nonsingular matrix B, the Jordan canonical form of Q v is \(BQ^{v}B^{-1}=\mathcal{J}=\operatorname{diag}(\mathcal{J}_{1}(d_{1}), \mathcal{J}_{2}(d_{2}), \ldots, \mathcal{J}_{p}(d_{p}) )\) and

is a n k ×n k matrix and n 1+n 2+⋯+n p =J+2. The matrix exponential exp(Q v t) can be computed [37] as

and in this case \(f(\mathcal{J}_{k}(d_{k}))\) takes the form

Numerically, the Jordan decomposition can be obtained through the MATLAB function jordan for a given Q v and the construction of (31) and hence the transition probability matrix P(t) can be easily computed in MATLAB. Other methods for computing matrix exponentials are reviewed in Moler and Van Loan [38]. Another numerically stable approach is the method of scaling and squaring [39], which has been employed by MATLAB function expm based on an optimal approach [40]. We used this function in sample size calculations for the trial design in cancer metastatic to bone in Sect. 6.
Supplementary Materials
The software for sample size calculations using the proposed method is available from the first author upon request.
Rights and permissions
About this article
Cite this article
Wu, L., Cook, R.J. The Design of Intervention Trials Involving Recurrent and Terminal Events. Stat Biosci 5, 261–285 (2013). https://doi.org/10.1007/s12561-013-9083-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12561-013-9083-z