Abstract
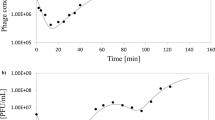
Latent period, burst time, and burst size, kinetic parameters of phage infection characteristic of a given phage/host system, have been measured for a wide variety of lactic acid bacteria. However, most studies to date were conducted in optimal growth conditions of host bacteria and did not consider variations due to changes in external factors. In this work, we determined the effect of temperature, pH, and starvation on kinetic parameters of phages infecting Lactobacillus paracasei, Lactobacillus plantarum, and Leuconostoc mesenteroides. For kinetics assessment, one-step growth curves were carried out in MRS broth at optimal conditions (control), lower temperature, pH 6.0 and 5.0 (MRS6 and MRS5, respectively), or in medium lacking carbon (MRSN) or nitrogen (MRSC) sources. Phage infection was progressively impaired as environmental conditions were modified from optimal. At lower temperature or pH, infection was delayed, as perceived by longer latent and burst times. Burst size, however, was lower, equal or higher than for controls, but this effect was highly dependent on the particular phage–host system studied. Phage infection was strongly inhibited in MRSC, but only mildly impaired in MRSN. Nevertheless, growth of all the bacterial strains tested was severely compromised by starvation, without significant differences between MRSC and MRSN, indicating that nitrogen compounds are specifically required for a successful phage infection, beyond their influence on bacterial growth.


Similar content being viewed by others
References
Abedon, S. (1989). Selection for bacteriophage latent period length by bacterial density: A theoretical examination. Microbial Ecology, 18(2), 79–88.
Arendt, E. K., Lonvaud, A., & Hammes, W. P. (1991). Lysogeny in Leuconostoc oenos. Journal of General Microbiology, 137(9), 2135–2139.
Baranyi, J., & Roberts, T. A. (1994). A dynamic approach to predicting bacterial growth in food. International Journal of Food Microbiology, 23(3–4), 277–294.
Binetti, A. G., Quiberoni, A., & Reinheimer, J. (2002). Phage adsorption to Streptococcus thermophilus: Influence of environmental factors and characterization of cell receptors. Food Research International, 35, 73–83.
Briggiler Marco, M., Garneau, J. E., Tremblay, D., Quiberoni, A., & Moineau, S. (2012). Characterization of two virulent phages of Lactobacillus plantarum. Applied and Environmental Microbiology, 78(24), 8719–8734.
Briggiler Marco, M., Reinheimer, J., & Quiberoni, A. (2015). Phage adsorption and lytic propagation in Lactobacillus plantarum: Could host cell starvation affect them? BMC Microbiology, 15, 273.
Briggiler Marcó, M., Reinheimer, J. A., & Quiberoni, A. (2010). Phage adsorption to Lactobacillus plantarum: Influence of physiological and environmental factors. International Journal of Food Microbiology, 138(3), 270–275.
Capra, M. L., Guglielmotti, D., Reinheimer, J., & Quiberoni, A. (2016). Bacteriophage: Biological aspects. In Reference module in food science. Cambridge: Elsevier.
Capra, M. L., Mercanti, D. J., Reinheimer, J. A., & Quiberoni, A. L. (2010). Characterisation of three temperate phages released from the same Lactobacillus paracasei commercial strain. International Journal of Dairy Technology, 63(3), 396–405.
Capra, M. L., Quiberoni, A., Ackermann, H. W., Moineau, S., & Reinheimer, J. A. (2006a). Characterization of a new virulent phage (MLC-A) of Lactobacillus paracasei. Journal of Dairy Science, 89(7), 2414–2423.
Capra, M. L., Quiberoni, A., & Reinheimer, J. (2006b). Phages of Lactobacillus casei/paracasei: Response to environmental factors and interaction with collection and commercial strains. Journal of Applied Microbiology, 100(2), 334–342.
Caso, J. L., de Los Reyes Gavilan, C., Herrero, M., Montilla, A., Rodriguez, A., & Suarez, J. E. (1995). Isolation and characterization of temperate and virulent bacteriophages of Lactobacillus plantarum. Journal of Dairy Science, 78, 741–750.
De Antoni, G., Zago, M., Vasek, O., Giraffa, G., Carminati, D., Marco, M. B., et al. (2010). Lactobacillus plantarum bacteriophages isolated from Kefir grains: Phenotypic and molecular characterization. Journal of Dairy Research, 77(1), 7–12.
Golec, P., Karczewska-Golec, J., Los, M., & Wegrzyn, G. (2014). Bacteriophage T4 can produce progeny virions in extremely slowly growing Escherichia coli host: Comparison of a mathematical model with the experimental data. FEMS Microbiology Letters, 351(2), 156–161.
Golec, P., Wiczk, A., Los, J., Konopa, G., Wegrzyn, G., & Los, M. (2011). Persistence of bacteriophage T4 in a starved Escherichia coli culture: Evidence for the presence of phage subpopulations. Journal of General Virology, 92, 997–1003.
Guglielmotti, D. M., Mercanti, D. J., & Briggiler Marcó, M. (2012). Infective cycle of dairy bacteriophages. In A. Quiberoni & J. A. Reinheimer (Eds.), Bacteriophages in Dairy Processing (pp. 99–122, Advances in Food Safety and Food Microbiology). Hauppauge, New York, USA: Nova Science Publishers.
Hemme, D. (2012). Leuconostoc and its use in dairy technology. In Handbook of animal-based fermented food and beverage technology (2nd ed., pp. 73–108). Boca Raton: CRC Press.
Lillehaug, D. (1997). An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. Journal of Applied Microbiology, 83(1), 85–90.
Lu, Z., Breidt, F., Jr., Fleming, H. P., Altermann, E., & Klaenhammer, T. R. (2003). Isolation and characterization of a Lactobacillus plantarum bacteriophage, phiJL-1, from a cucumber fermentation. International Journal of Food Microbiology, 84(2), 225–235.
Mercanti, D. J., Ackermann, H. W., & Quiberoni, A. (2015). Characterization of two temperate Lactobacillus paracasei bacteriophages: Morphology, kinetics and adsorption. Intervirology, 58(1), 49–56.
Mercanti, D. J., Carminati, D., Reinheimer, J. A., & Quiberoni, A. (2011). Widely distributed lysogeny in probiotic lactobacilli represents a potentially high risk for the fermentative dairy industry. International Journal of Food Microbiology, 144(3), 503–510.
Mercanti, D. J., Rousseau, G. M., Capra, M. L., Quiberoni, A., Tremblay, D. M., Labrie, S. J., et al. (2016). Genomic diversity of phages infecting probiotic strains of Lactobacillus paracasei. Applied and Environmental Microbiology, 82(1), 95–105.
Müller-Merbach, M., Kohler, K., & Hinrichs, J. (2007). Environmental factors for phage-induced fermentation problems: Replication and adsorption of the Lactococcus lactis phage P008 as influenced by temperature and pH. Food Microbiology, 24(7–8), 695–702.
Nes, I. F., Brendehaug, J., & von Husby, K. O. (1988). Characterization of the bacteriophage B2 of Lactobacillus plantarum ATCC 8014. Biochimie, 70(3), 423–427.
Nowicki, D., Kobiela, W., Węgrzyn, A., Węgrzyn, G., & Szalewska-Pałasz, A. (2013). ppGpp-dependent negative control of DNA replication of Shiga toxin-converting bacteriophages in Escherichia coli. Journal of Bacteriology, 195(22), 5007–5015.
Potrykus, K., & Cashel, M. (2008). (p)ppGpp: Still magical? Annual Review of Microbiology, 62, 35–51.
Pujato, S. A., Mercanti, D. J., Guglielmotti, D. M., Rousseau, G. M., Moineau, S., Reinheimer, J. A., et al. (2015). Phages of dairy Leuconostoc mesenteroides: Genomics and factors influencing their adsorption. International Journal of Food Microbiology, 201, 58–65.
Quiberoni, A., & Reinheimer, J. (1998). Physicochemical characterization of phage adsorption to Lactobacillus helveticus ATCC 15807 cells. Journal of Applied Microbiology, 85, 762–768.
Saarela, M., Mogensen, G., Fondén, R., Mättö, J., & Mattila-Sandholm, T. (2000). Probiotic bacteria: Safety, functional and technological properties. Journal of Biotechnology, 84(3), 197–215.
Server-Busson, C., Foucaud, C., & Leveau, J.-Y. (1999). Selection of dairy Leuconostoc isolates for important technological properties. Journal of Dairy Research, 66(2), 245–256.
Sozzi, T., Poulin, J. M., Maret, R., & Pousaz, R. (1978). Isolation of a bacteriophage of Leuconostoc mesenteroides from dairy products. Journal of Applied Bacteriology, 44(1), 159–161.
Suarez, V., Moineau, S., Reinheimer, J., & Quiberoni, A. (2008). Argentinean Lactococcus lactis bacteriophages: Genetic characterization and adsorption studies. Journal of Applied Microbiology, 104(2), 371–379.
Svensson, U., & Christiansson, A. (1991). Methods for phage monitoring. Bulletin—FIL-IDF, 263, 29–39.
Trucco, V., Reinheimer, J., Quiberoni, A., & Suarez, V. B. (2011). Adsorption of temperate phages of Lactobacillus delbrueckii strains and phage resistance linked to their cell diversity. Journal of Applied Microbiology, 10(10), 1365–2672.
Wang, I.-N., Dykhuizen, D., & Slobodkin, L. (1996). The evolution of phage lysis timing. Evolutionary Ecology, 10(5), 545–558.
Zhang, X., Lan, Y., Jiao, W., Li, Y., Tang, L., Jiang, Y., et al. (2015). Isolation and characterization of a novel virulent phage of Lactobacillus casei ATCC 393. Food and Environmental Virology, 7(4), 333–341.
Acknowledgements
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET; Project PIP 112-201201-00046; Argentina) and by the Universidad Nacional del Litoral (UNL; Project CAI+D PI 501-201101-00039; Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zaburlin, D., Quiberoni, A. & Mercanti, D. Changes in Environmental Conditions Modify Infection Kinetics of Dairy Phages. Food Environ Virol 9, 270–276 (2017). https://doi.org/10.1007/s12560-017-9296-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9296-2