Abstract
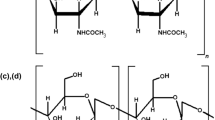
Enteric viruses are a major problem in the food industry, especially as human noroviruses are the leading cause of nonbacterial gastroenteritis. Chitosan is known to be effective against some enteric viral surrogates, but more detailed studies are needed to determine the precise application variables. The main objective of this work was to determine the effect of increasing chitosan concentration (0.7–1.5 % w/v) on the cultivable enteric viral surrogates, feline calicivirus (FCV-F9), murine norovirus (MNV-1), and bacteriophages (MS2 and phiX174) at 37 °C. Two chitosans (53 and 222 kDa) were dissolved in water (53 kDa) or 1 % acetic acid (222 KDa) at 0.7–1.5 %, and were then mixed with each virus to obtain a titer of ~5 log plaque-forming units (PFU)/mL. These mixtures were incubated for 3 h at 37 °C. Controls included untreated viruses in phosphate-buffered saline and viruses were enumerated by plaque assays. The 53 kDa chitosan at the concentrations tested reduced FCV-F9, MNV-1, MS2, and phi X174 by 2.6–2.9, 0.1–0.4, 2.6–2.8, and 0.7–0.9 log PFU/mL, respectively, while reduction by 222 kDa chitosan was 2.2–2.4, 0.8–1.0, 2.6–5.2, and 0.5–0.8 log PFU/mL, respectively. The 222 kDa chitosan at 1 and 0.7 % w/v in acetic acid (pH 4.5) caused the greatest reductions of MS2 by 5.2 logs and 2.6 logs, respectively. Overall, chitosan treatments showed the greatest reduction of MS2, followed by FCV-F9, phi X174, and MNV-1. These two chitosans may contribute to the reduction of enteric viruses at the concentrations tested but would require use of other hurdles to eliminate food borne viruses.

Similar content being viewed by others
References
Badawy, M., & Rabea, E. I. (2009). Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biology and Technology, 51, 110–117.
Bae, J., & Schwab, K. J. (2008). Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Applied and Environmental Microbiology, 74, 477–484.
Bidawid, S., Farber, J. M., & Sattar, S. A. (2000). Contamination of foods by food handlers: Experiments on hepatitis A virus transfer to food and its interruption. Applied and Environmental Microbiology, 66, 2759–2763.
Bozkurt, H., D’Souza, D. H., & Davidson, P. M. (2014). Thermal inactivation of human norovirus surrogates in spinach and measurement of its uncertainty. Journal of Food Protection, 77(2), 276–283.
Brentlinger, K. L., Hafenstein, S., Novak, C. R., Fane, B. A., Borgon, R., McKenna, R., & Agbandje-McKenna, M. (2002). Microviridae, a family divided: Isolation, characterization, and genome sequence of phi MH2K, a bacteriophage of the obligate intracellular parasitic bacterium Bdellovibriobacteriovirus. Journal of Bacteriology, 184, 1089–1094.
Cannon, J. L., Papafragkou, E., Park, G. W., Osborne, J., Jaykus, L. A., & Vinje, J. (2006). Surrogates for the study of norovirus stability and inactivation in the environment: A comparison of murine norovirus and feline calicivirus. Journal of Food Protection, 69, 2761–2765.
Chirkov, S. N. (2002). The antiviral activity of chitosan (review). Applied Biochemistry and Microbiology, 38, 1–8.
Cromeans, T., Park, G. W., Costantini, V., Lee, D., Wang, Q., Farkas, T., et al. (2014). Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Applied and Environmental Microbiology, 80(18), 5743–5751.
Davis, R., Zivanovic, S., D’Souza, D. H., & Davidson, P. M. (2012). Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiology, 32, 57–62.
Devlieghere, F., Vermeulen, A., & Debevere, J. (2004). Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiology, 21, 703–714.
D’Souza, D. H., Sair, A., Williams, K., Papafragkou, E., Jean, J., Moore, C., & Jaykus, L. (2006). Persistence of caliciviruses on environmental surfaces and their transfer to food. International Journal of Food Microbiology, 108, 84–91.
El Ghaouth, A., Arul, J., Asselin, A., & Benhamou, N. (1992). Antifungal activity of chitosan on post-harvest pathogens: Induction of morphological and cytological alterations in Rhizopus stolonifer. Mycological Research, 96, 769–779.
Hall, A. J., Lopman, B. A., Payne, D. C., Patel, M. M., Gastanaduy, P. A., Vinje, J., & Parashar, U. D. (2013). Norovirus disease in the United States. Emerging Infectious Disease, 19, 1198–1205.
Hall, A. J., Wikswo, M. E., Pringle, K., Gould, L. H., & Parashar, U. D. (2014). Vital signs: Foodborne norovirus outbreaks—United States, 2009-2012. Morbidity and Mortality Weekly Report, 63, 491–495.
Helander, I. M., Nurmiaho-Lassila, E. L., Ahvenainen, R., Rhoades, J., & Roller, S. (2001). Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. International Journal of Food Microbiology, 71, 235–244.
Kochkina, Z. M., & Chirkov, S. N. (2000). Effect of chitosan derivatives on the reproduction of coliphages T2 and T7. Microbiology, 69, 208–211.
Kong, M., Chen, X. G., Liu, C. S., Liu, C. G., Meng, X. H., & Yu, L. J. (2008). Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surfaces B: Biointerfaces, 65, 197–202.
Langlet, J., Gaboriaud, F., & Gantzer, C. (2007). Effects of pH on plaque forming unit counts and aggregation of MS2 bacteriophage. Journal of Applied Microbiology, 103, 1632–1638.
Liu, H., Du, Y. M., Wang, X. H., & Sun, L. P. (2004). Chitosan kills bacteria through cell membrane damage. International Journal of Food Microbiology, 95, 147–155.
Liu, J., Tian, S., Meng, X., & Xu, Y. (2007). Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biology and Technology, 44, 300–306.
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., et al. (1999). Food-related illness and death in the United States. Emerging Infectious Diseases, 5, 607–625.
Pospieszny, H., Chirkov, S., & Atabekov, J. (1991). Induction of antiviral resistance in plants by chitosan. Plant Science, 79, 63–68.
Rabea, E. I., Badawy, M. E., Stevens, C. V., Smagghe, G., & Steurbaut, W. (2003). Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules, 4, 1457–1465.
Reddy, M. V. B., Belkacemi, K. R., Corcuff, R., Castaigne, F., & Arul, J. (2000). Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biology and Technology, 20, 39–51.
Sair, A. I., D’Souza, D. H., & Jaykus, L. A. (2002a). Human enteric viruses as causes of foodborne disease. Comprehensive Reviews in Food Science and Food Safety, 1, 73–89.
Sair, A. I., D’Souza, D. H., Moe, C. L., & Jaykus, L. A. (2002b). Improved detection of human enteric viruses in foods by RT-PCR. Journal of Virological Methods, 100, 57–69.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Disease, 17(1), 7–15.
Seyfarth, F., Schliemann, S., Elsner, P., & Hipler, U. C. (2008). Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against Candida albicans, Candida krusei and Candida glabrata. International Journal of Pharmaceutics, 353, 139–148.
Shahidi, F., Arachchi, J. K. V., & Jeon, Y.-J. (1999). Food applications of chitin and chitosans. Trends in Food Science and Technology, 10, 37–51.
Su, X., Zivanovic, S., & D’Souza, D. H. (2009). Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. Journal of Food Protection, 72, 2623–2628.
Sudarshan, N. R., Hoover, D. G., & Knorr, D. (1992). Antibacterial action of chitosan. Food Biotechnology, 6, 257–272.
Wang, G. H. (1992). Inhibition and inactivation of 5 species of foodborne pathogens by chitosan. Journal of Food Protection, 55, 916–919.
Wobus, C. E., Thackray, L. B., & Virgin, H. W. (2006). Murine norovirus: a model system to study norovirus biology and pathogenesis. Journal of Virology, 80, 5104–5112.
Zheng, L.-Y., & Zhu, J.-F. (2003). Study on antimicrobial activity of chitosan with different molecular weights. Carbohydrate Polymers, 54, 527–530.
Acknowledgments
The authors gratefully acknowledge the funding for this research that was provided by the Tennessee Agricultural Experiment Station Hatch Fund (TEN 398) and UT Innovation Grants program funding to D. D’Souza and S. Zivanovic. The authors gratefully acknowledge the assistance provided by Primex for the 222 kDa chitosan used in the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
As this article does not contain any studies with human participants or animals performed by any of the authors, informed consent was not required.
Rights and permissions
About this article
Cite this article
Davis, R., Zivanovic, S., Michael Davidson, P. et al. Enteric Viral Surrogate Reduction by Chitosan. Food Environ Virol 7, 359–365 (2015). https://doi.org/10.1007/s12560-015-9208-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-015-9208-2