Abstract
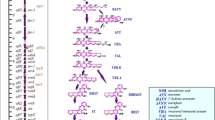
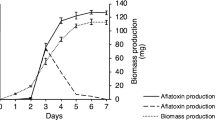
The influence of varying combinations of water activity (aw) and temperature on growth, aflatoxin biosynthesis and aflR/aflS expression of Aspergillus parasiticus was analysed in the ranges 17–42°C and 0.90–0.99 aw. Optimum growth was at 35°C. At each temperature studied, growth increased from 0.90 to 0.99 aw. Temperatures of 17 and 42°C only supported marginal growth. The external conditions had a differential effect on aflatoxin B1 or G1 biosynthesis. The temperature optima of aflatoxin B1 and G1 were not at the temperature which supported optimal growth (35°C) but either below (aflatoxin G1, 20–30°C) or above (aflatoxin B1, 37°C). Interestingly, the expression of the two regulatory genes aflR and aflS showed an expression profile which corresponded to the biosynthesis profile of either B1 (aflR) or G1 (aflS). The ratios of the expression data between aflS:aflR were calculated. High ratios at a range between 17 and 30°C corresponded with the production profile of aflatoxin G1 biosynthesis. A low ratio was observed at >30°C, which was related to aflatoxin B1 biosynthesis. The results revealed that the temperature was the key parameter for aflatoxin B1, whereas it was water activity for G1 biosynthesis. These differences in regulation may be attributed to variable conditions of the ecological niche in which these species occur.



Similar content being viewed by others
References
Bennett JW, Christensen SB (1983) New perspectives on aflatoxin biosynthesis. Adv Appl Microbiol 29:53–92
Bhatnagar D, Carry JW, Ehrlich K, Yu J, Cleveland TE (2006) Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia 162:155–166
Cai J, Zeng H, Shima Y, Hatabayashi H, Nakagawa H, Ito Y, Adachi Y, Nakajima H, Yabe K (2008) Involvement of the nadA gene in formation of G-group aflatoxins in Aspergillus parasiticus. Fungal Genet Biol 45:1081–1093
Chang PK (2003) The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol Gen Genomics 268:711–719
Du W, Obrian R, Payne GA (2007) Function and regulation of aflJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Add Contam 24:1043–1050
Ehrlich KC (2009) Predicted roles of the uncharacterized clustered genes in aflatoxin biosynthesis. Toxins 1:37–58
Ehrlich K, Cary JW (1995) An aflatoxin biosynthesis regulatory protein (AFLR) is a sequence-specific DNA binding protein. Fungal Genet Newsl 42A:57
Ehrlich KC, Montalbano BG, Cary JW (1999) Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene 230:249–257
Faraj MK, Smith JE, Harran G (1991) Interaction of water activity and temperature on aflatoxin production by Aspergillus flavus and A. parasiticus in irradiated maize seeds. Food Add Contam 8:731–736
Giorni P, Magan N, Pietri A, Bertuzzi T, Battilani P (2007) Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int J Food Microbiol 113:330–338
Gqaleni N, SmithJE LJ, Gettinby G (1997) Effects of temperature, water activity, and incubation time on production of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface agar culture. Appl Environ Microbiol 63:1048–1053
Jurado M, Marín P, Magan N, González-Jaén MT (2008) Relationship between solute and matric potential stress, temperature, growth and FUM1 gene expression in two Fusarium verticillioides strains from Spain. Appl Environ Microbiol 74:2032–2036
Lin YC, Ayres JC, Koehler PE (1980) Influence of temperature cycling on the production of aflatoxins B1 and G1 by Aspergillus parasiticus. Appl Environ Microbiol 40:333–336
Magan N, Aldred D (2007a) Why do fungi produce mycotoxins? In: Dijksterhuis J, Samson RA (eds) Food mycology: a multifaceted approach to fungi and food. Taylor & Francis, Boca Raton, Fla, pp 121–133
Magan N, Aldred D (2007b) Environmental fluxes and fungal interactions: maintaining a competitive edge. In: van West P, Avery S, Stratford M (eds) Stress in yeasts and filamentous fungi, Chapter 2. Elsevier, Amsterdam, pp 19–35
O’Brian GR, Georgianna DR, Wilkinson JR, Yu J, Abbas HK, Bhatnagar D, Cleveland TE, Nierman W, Payne GA (2007) The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia 99:232–239
Park KY, Bullerman LB (1981) Increased aflatoxin production by Aspergillus parasiticus under conditions of cycling temperatures. J Food Sci 46:1147–1151
Pitt JI, Hocking AD (1999) Fungi and food spoilage, 2nd edn.Aspen, Gaithersburg
Samapundo S, De Meulenaer B, Atukwase A, Debevere J, Devlieghere F (2007) The influence of modified atmospheres and their interaction with water activity on the radial growth and fumonisin B1 production of Fusarium verticillioides and F. proliferatum on corn. Part II: The effect of initial headspace oxygen concentration. Int J Food Microbiol 113:339–345
Schmidt-Heydt M, Magan N, Geisen R (2008) Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol Lett 284:142–149
Schmidt-Heydt M, Abdel-Hadi A, Magan N, Geisen R (2009) Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int J Food Microbiol 135:231–237
Sorensen WG, Hesseltine CW, Shotwell OL (1967) Effect of temperature on production of aflatoxin on rice by A. flavus. Mycopathologia 33:49–55
Yu J, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW (2004) Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol 70:1253–1262
Acknowledgement
We would like to thank Katja Kramer and Sabine Häckel for excellent technical assistance. This work was supported by the EU project EC KBBE-2007-222690-2 MYCORED.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt-Heydt, M., Rüfer, C.E., Abdel-Hadi, A. et al. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotox Res 26, 241–246 (2010). https://doi.org/10.1007/s12550-010-0062-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-010-0062-7