Abstract
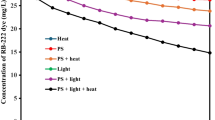
In this paper, the optimum process parameters were obtained through treating phenol of simulated semi-coking wastewater using heat, Fe2+, Fe0 and semi-coke to catalyze persulfate. The results of phenol decomposition using PS catalyzed by heating, Fe2+, Fe0 and semi-coke were compared for selecting a better activating way. The article investigated the effects of temperature, catalyzer dosage, pH value and reaction time. The experiment showed the four methods can all catalyzed the process. Under the experimental conditions of heating, Fe2+, Fe0 and semi-coke degradation rate could reach to 20.7%, 75.1%, 94.5% and 40.0%, respectively. On this basis, this study established an L16(45) table to analyze the main influencing factors in semi-coke/Fe0 catalyzing system. Under the optimum conditions, the degradation rate of Phenol reached to 93.6%. However, the PS dosage was reduced by 14.4%.
Similar content being viewed by others
References
Arturo R, Aurora S, Fernando V, Concepción G, 2010. Diuron abatement using activated persulphate: Effect of pH, Fe (II) and oxidant dosage. Chemical Engineering Journal, 162: 257–265.
Cao J S, Zhang W X, Brown D G, 2008. Oxidation of lindane with Fe(II) — Activated sodium persulfate. Environmental Science and Technology, 25(2): 221–228.
Georgi A, Kopinke F D, 2005. Interaction of adsorption and catalytic reactions in water decontamination processes: Part 1. Oxidation of organic contaminants with hydrogen peroxide catalyzed by active carbon. Applied Catalysis B: Environmental, 58(1–2): 9–18.
Guedes A M F M, Madeira L M P, Boaventura R A R, 2003. Fention oxidation of cork cooking waster water: overall kinetic analysis. Water Research, 37(13): 3061–3069.
Hori H, Murayama M, Inoue N, 2010. Efficient mineralization of hydro perfluorocarb oxylicacids with persulfate in hot water. Catalysis Today, 151(1–2): 131–136.
Huang Y, Wang L, Huang Y H, Wang Y L, Shi K, 2008. Polymeric ferric sulfate treat chemical plating Ni-P liquid waste. Technology of Water Treatment, 34(2): 76–78.
Kimura M, Miyamoto I, 1994. Discovery of the activated carbon radical GAC+ and the novel oxidation reactions comprising the GAC/GAC+ cycle as a catalyst in an aqueous solution. Bulletin of the Chemical Society of Japan, 67(9): 2357–2360.
Lv Y T, Wang L, Chen Z, Sun T, Wu H Y, Wang T, Wang Z Y, 2010. Research on the pretreatment of semi-coking wastewater by Fenton oxidation combined with ammonia stripping. Industrial Water Treatment, 30(11): 56–58.
Neta P, Huie R E, Ross A B, 1998. Rate constants for reactions of inorganic radicals in aqueous solution. Journal of Physical and Chemical Reference Data, 17(3): 221–228.
Oh S Y, Kang S G, Chiu P C, 2010. Degradation of 2, 4-dinitrotoluene by persulfate activated with zero-valent iron. Science of the Total Environmental, 408(16): 3464–3468.
Salari D, Niaei A, Aber S, 2009. The photooxidation destruction of C.I. Basic Yellow 2 using UV/S2O 82− process in a rectangular continuous photo reactor. Journal of Hazardous Materials, 166(1): 61–66.
Shan J J, Du Z L, Li Q, 2009. Application of ultrasonic in chemical industry. Hebei Industrial Science and Technology, 26(2): 127–130.
Shen P, Wang X Q, 2012. Study on semi-coke preparation activated carbon. Coal Converse, (2): 89–94.
Waldemer R H, Tratnyek P G, Johnson R L, 2007. Oxidation of chlorinated ethense by heat-activated persulfate: Kinetics and products. Environmental Science and Technology, 41(3): 1010–1015.
Wang B, Li J, 2012. Based on sulfate radical advanced oxidation technology research and application. Environmental Engineering, 30(8): 53–57.
Wang H Z, 2012. Analysis of countermeasures of the development of coal logistics in Northern Shaanxi. Value Engineering, 16(15): 16–17.
Xu S R, Shi C L, Chen H L, 2011. Microelectrolysis and AO biochemical method in low temperature environment. Environmental Science & Technology, 34(8): 5–8.
Yang J Y, 2011. Improvement of determining volatile phenol in wastewater by using 4-aminoantioyrine direct colorimetric method. Environmental Science and Management, 36(8): 36–39.
Yang S Y, Chen Y Y, Xu H Z, 2008. Activated persulfate advanced oxidation technology. Progress of Chemistry, 20(9): 1433–1438.
Yang S Y, Wang P, Yang X, 2010. Degradation efficiencies of azodye acid orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. Journal of Hazardous Materials, 179(1–3): 552–558.
Zhao J Y, Zhang Y B, Quan X, 2010. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zerovalent iron and peroxydisulfate at ambient temperature. Separation and Purification Technology, 71(3): 302–307.
Zhou C L, Xue S K, 2007. Preliminary discussion on China coking waste water treatment technology development and application. Environmental Protection of Coal Mine, 10(3): 79–81.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, ZN., Zhang, KK. & Liu, YM. Experimental research on oxidation of phenol by activated persulfate. J Coal Sci Eng China 19, 560–565 (2013). https://doi.org/10.1007/s12404-013-0419-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12404-013-0419-6