Abstract
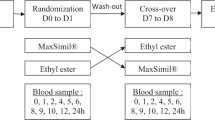
The aim of this study was to assess baseline levels and changes in plasma fatty acid profiles in children and adolescents with ADHD, in a placebo-controlled study with Omega 3/6 supplementation, and to compare with treatment response. Seventy-five children and adolescents aged 8–18 years with DSM-IV ADHD were randomized to 3 months of Omega 3/6 (Equazen eye q) or placebo, followed by 3 months of open phase Omega 3/6 for all. n-3, n-6, n-6/n-3 ratio, EPA and DHA in plasma were measured at baseline, 3 and 6 months. Subjects with more than 25 % reduction in ADHD symptoms were classified as responders. At baseline, no significant differences in mean fatty acid levels were seen across active/placebo groups or responder/non-responder groups. The 0–3 month changes in all parameters were significantly greater in the active group (p < 0.01). Compared to non-responders, the 6-month responders had significantly greater n-3 increase at 3 months and decrease in n-6/n-3 ratio at 3 and 6 months (p < 0.05). Omega 3/6 supplementation had a clear impact on fatty acid composition of plasma phosphatidyl choline in active versus placebo group, and the fatty acid changes appear to be associated with treatment response. The most pronounced and long-lasting changes for treatment responders compared to non-responders were in the n-6/n-3 ratio.
Similar content being viewed by others
References
Chen JR, Hsu SF, Hsu CD et al (2004) Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem 15:467–472
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998) ADHD rating scale-IV: checklists, norms, and clinical interpretations. Guilford, New York
Germano M, Meleleo D, Monorfano G, Adorni L, Negroni M, Berra B et al (2007) Plasma, red blood cell phospholipids and clinical evaluation after long chain omega-3 supplementation in children with attention deficit hyperactivity disorder (ADHD). Nutr Neurosci 10:1–9
Gustafsson P, Birberg-Thornberg U, Duchén K, Landgren M, Malmberg K, Pelling H et al (2010) EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr 99:1540–1549
Guy W (1976) ECDEU Assessment manual for psychopharmacology. Revised. Bethesda (MD), US Department of health, education, and welfare
Haag M (2003) Essential fatty acids and the brain. Can J Psychiatry 48:195–203
Innis SM (2007) Dietary (n-3) fatty acids and brain development. J Nutr 137:855–859
Johnson M, Östlund S, Fransson G, Kadesjö B, Gillberg C (2009) Omega-3/Omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Att Disorders 12:394–401
Landen M, Davidsson P, Gottfries C-G et al (2002) Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizofrenia. Schizophrenia Res 55:83–88
Percy P, Vilbergsson G, Percy A et al (1991) The fatty acid composition of placenta in intrauterine growth retardation. Biochim Biophys Acta 1084:173–177
Richardson AJ, Montgomery P (2005) The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics 115:1360–1366
Richardson AJ, Puri BK (2002) A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuro-Psychopharmacol Biol Psychiatry 26:233–239
Sinn N, Bryan J (2007) Effect of supplementation with polyunsaturated fatty acids and micronutrients on ADHD-related problems with attention and behaviour. J Dev Behav Pediatr 28:82–91
Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR et al (1995) Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr 62:761–768
Acknowledgments
The study was supported by grants from Equazen/Vifor Pharma, Wilhelm and Martina Lundgrens Vetenskapsfond and by the Swedish MRC (grant no 2,006–3,449). Funders have not been involved in study design, data collection or analysis, manuscript preparation or publication decisions. Preliminary results were reported as poster and oral presentation at the 3rd International Congress on ADHD, Berlin, Germany, May 26–29, 2011. Dr. Johnson has received honoraria from Vifor Pharma for Advisory Board participation and lectures.
Conflict of interest
All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrails.gov id: no: NCT01219309. http://clinicaltrials.gov/ct2/show/NCT01219309?term=omega+3%2F6&rank=1
Rights and permissions
About this article
Cite this article
Johnson, M., Månsson, JE., Östlund, S. et al. Fatty acids in ADHD: plasma profiles in a placebo-controlled study of Omega 3/6 fatty acids in children and adolescents. ADHD Atten Def Hyp Disord 4, 199–204 (2012). https://doi.org/10.1007/s12402-012-0084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12402-012-0084-4