Abstract
Introduction
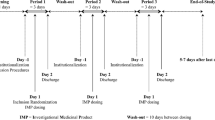
Clopacin® (Acino Pharma AG) is a proprietary, besylate salt and lactose-free formulation of the widely-used anti-platelet treatment, clopidogrel. This study aimed to evaluate the bioequivalence of Clopacin® with the originator as reference drug, using a guideline-compliant trial design: open-labeled, randomized, single-dose (clopidogrel 75 mg tablet), two-period, crossover trial in 48 healthy male volunteers, with a 7 day wash-out period.
Methods
Plasma samples were collected at intervals and extracted before quantifying clopidogrel concentrations using a fully validated LC–MS/MS method. Bioequivalence of Clopacin® and the reference drug was established by comparison of the primary pharmacokinetic parameters, C max, AUC0–t, and AUC0–∞.
Results
The parameter values were similar for the two products (analysis of variance) and provided Clopacin/reference ratios (least squares means) of >90% and 90% confidence intervals (CIs 84.64–105.50%, 90.43–111.22%, 88.75–110.71%, respectively) that were well within the limits set for defining bioequivalence, according to international guidelines. The respective Clopacin® and reference drug values for mean time to maximal plasma clopidogrel concentration (t max) were 0.83 and 0.91 h, and for terminal elimination half-life were 3.99 and 3.51 h. The intra-subject coefficients of variability for maximal plasma clopidogrel concentration (C max), area under the plasma clopidogrel concentration versus time curve, at 48 h (AUC0–t) and extrapolated to infinity (AUC0–∞) were 32.2%, 30.2%, and 28.9% (least square means), respectively, and the respective power values were 99.5%, 97.1%, and 95.3%.
Conclusion
This bioequivalence study provided robust clopidogrel pharmacokinetic data that established the bioequivalence of Clopacin® and the reference originator drug.
Funding
Acino Pharma AG (formerly Cimex AG)

Similar content being viewed by others
References
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
Anon X. Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81–106.
Tsoumani ME, Ntalas IV, Goudevenos JA, Tselepis AD. Evaluating the bioequivalence of clopidogrel generic formulations. Curr Med Res Opin. 2015;31(5):861–4.
Cattaneo M. The platelet P2Y receptors as targets for new antithrombotic drugs. J Thromb Haemost. 2003;1(6):1133–5.
Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Hemost. 2005;31(2):174–83.
Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607–21.
Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–33.
Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352(12):1179–89.
Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–20.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502.
Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(14):1555–70.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344–426.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425.
Tsoumani ME, Kalantzi KI, Goudevenos IA, Tselepis AD. Clopidogrel generic formulations in the era of new antiplatelets: a systematic review. Curr Vasc Pharmacol. 2014;12(5):766–77.
EMA, CPMP/EWP/QWP/1401/98: Note for Guidance on the Investigation of Bioavailability and Bioequivalence—revision—came into operation in 2002. European Medical Agency 2001.
EMA, CPMP/EWP/QWP/1401/98 Rev. 1/Corr **: Guideline on the investigation of bioequivalence. European Medical Agency 2010.
FDA, Guidance for Industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. Food and Drug Administration, Center for Drug Evaluation and Research (CDER) 2013.
Elder DP, Delaney E, Teasdale A, Eyley S, Reif VD, Jacq K, et al. The utility of sulfonate salts in drug development. J Pharm Sci. 2010;99(7):2948–61.
Pawlowska M, Duda J, Tejchman-Malecka B, Bogiel M, Marzec A, Sieradzki E. Usefulness of the parent compound determination in bioequivalence evaluation of clopidogrel generic products. Arzneimittelforschung. 2009;59(6):289–96.
Paulekuhn GS, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection based on analysis of the Orange Book database. J Med Chem. 2007;50(26):6665–72.
Seo JH, Park JB, Choi WK, Park S, Sung YJ, Oh E, et al. Improved oral absorption of cilostazol via sulfonate salt formation with mesylate and besylate. Drug Des Devel Ther. 2015;9:3961–8.
Zupancic V, Smrkolj M, Benkic P, Simonic I, Plevnik M, Ritlop G, et al. Preformulation investigation of some clopidogrel addition salts. Acta Chim Slov. 2010;57(2):376–85.
European Union, Directive 2001/83/EC of The European Parliament and of the Council: Community Code relating to Medicinal Products for Human Use (as amended). 2012 Article 10, 2(b).
FDA, (US, Food and Drug Administration), 21CFR320.1(c). 2015.
Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84(5):891–6.
Caplain H, Donat F, Gaud C, Necciari J. Pharmacokinetics of clopidogrel. Semin Thromb Hemost. 1999;25(Suppl 2):25–8.
Lagorce P, Perez Y, Ortiz J, Necciari J, Bressolle F. Assay method for the carboxylic acid metabolite of clopidogrel in human plasma by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 1998;720(1–2):107–17.
Ksycinska H, Rudzki P, Bukowska-Kiliszek M. Determination of clopidogrel metabolite (SR26334) in human plasma by LC-MS. J Pharm Biomed Anal. 2006;41(2):533–9.
Souri E, Jalalizadeh H, Kebriaee-Zadeh A, Shekarchi M, Dalvandi A. Validated HPLC method for determination of carboxylic acid metabolite of clopidogrel in human plasma and its application to a pharmacokinetic study. Biomed Chromatogr. 2006;20(12):1309–14.
Lainesse A, Ozalp Y, Wong H, Alpan RS. Bioequivalence study of clopidogrel bisulfate film-coated tablets. Arzneimittelforschung. 2004;54(9A):600–4.
El Ahmady O, Ibrahim M, Hussein AM, Bustami RT. Bioequivalence of two oral formulations of clopidogrel tablets in healthy male volunteers. Int J Clin Pharmacol Ther. 2009;47(12):780–4.
Garces-Eisele J, Ruiz-Arguelles A, Estrada-Marin L, Reyes-Nunez V, Vazquez-Perez R, Guzman-Garcia O, et al. Pharmacogenetic selection of volunteers increases stringency of bioequivalence studies; the case of clopidogrel. Indian J Pharm Sci. 2014;76(4):281–6.
Kim SD, Kang W, Lee HW, Park DJ, Ahn JH, Kim MJ, et al. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin Ther. 2009;31(4):793–803.
Richter W, Erenmemisoglu A, Van der Meer MJ, Emritte N, Tuncay E, Koytchev R. Bioequivalence study of two different clopidogrel bisulfate film-coated tablets. Arzneimittelforschung. 2009;59(6):297–302.
Setiawati E, Yunaidi DA, Handayani LR, Santoso ID, Setiawati A, Tjandrawinata RR. Bioequivalence study of two clopidogrel film-coated tablet formulations in healthy volunteers. Arzneimittelforschung. 2011;61(12):681–4.
ICH, ICH harmonised tripartite guideline: Validation of analytical procedures: Text and methodology Q2(R1) (subsequently updated in 2005 as ICH guideline Q2B) 1996.
Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS 2nd, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5(12):2429–36.
Kim BH, Kim JR, Lim KS, Shin HS, Yoon SH, Cho JY, et al. Comparative pharmacokinetics/pharmacodynamics of clopidogrel besylate and clopidogrel bisulfate in healthy Korean subjects. Clin Drug Investig. 2012;32(12):817–26.
Taubert D, Kastrati A, Harlfinger S, Gorchakova O, Lazar A, von Beckerath N, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost. 2004;92(2):311–6.
Steinhubl SR. Genotyping, clopidogrel metabolism, and the search for the therapeutic window of thienopyridines. Circulation. 2010;121(4):481–3.
Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360(4):363–75.
EMA, EMA website: http://www.ema.europa.eu/ema/.
Swissmedic, Swissmedic website: http://www.swissmedicinfo.ch/.
Acknowledgments
The trial was sponsored by Acino Pharma AG (formerly Cimex AG); the study was conducted by Lambda Therapeutic Research Ltd., Premier House-l, Gandhinagar-Sarkhej Highway, Bodakdev, Ahmedabad- 380 054, Gujarat, India, under contract from Acino Pharma AG (formerly Cimex AG. All the data reported here were provided to Acino Pharma AG (formerly Cimex AG) by Lambda Therapeutic Research Ltd., Premier House-l, Gandhinagar-Sarkhej Highway, Bodakdev, Ahmedabad- 380 054, Gujarat, India, under contract from Acino Pharma AG (formerly Cimex AG). The article processing charges for this publication were funded by Acino Pharma AG. The named author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, takes responsibility for the integrity of the work as a whole, and has given final approval for the version to be published. The author had full access to all relevant aggregated study data required to understand and report research findings for this study and takes complete responsibility for the integrity of such data and the accuracy of the data analysis. The study protocol and clinical study report were submitted to the journal by Acino Pharma AG (formerly Cimex AG) in order to make them available to the journal Editor and the Reviewers. The manuscript was written by the author without any additional medical writing or editorial assistance.
Disclosures
Gerard P. McGregor receives consultancy fees from Acino Pharma AG and received a fee for writing this article.
Compliance with Ethics Guidelines
Ethical approval of the study protocol, the informed consent form and other appendices was provided by an independent ethics committee (in Suraksha on 10 March, 2006), which was kept informed of all the adverse events occurring during the study. The study was compliant with the 1996 version of ICH guidelines for good clinical practice (GCP) and conducted according to a number of ethical guidelines for medical research on human subjects; those of the Declaration of Helsinki, of the World Medicine Association (WMA) (Tokyo, 2004) and of the Indian Council of Medical Research (1980). Informed consent was obtained from all subjects for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McGregor, G.P. Pivotal Bioequivalence Study of Clopacin®, a Generic Formulation of Clopidogrel 75 mg Film-Coated Tablets. Adv Ther 33, 186–198 (2016). https://doi.org/10.1007/s12325-016-0290-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0290-0