Abstract
Introduction
The disease characteristics of multiple sclerosis (MS) appear to differ between Hispanic and Caucasian patients, with Hispanic patients having a younger age at onset, and a higher prevalence of optic nerve and spinal cord involvement. Fingolimod, the first-in-class oral sphingosine 1-phosphate receptor modulator approved for the treatment of relapsing MS, has been shown to significantly reduce annualized relapse rates (ARRs), lesion-based magnetic resonance imaging (MRI) activity, confirmed disability, and brain volume loss, compared with placebo or intramuscular interferon beta-1a (IFNβ-1a IM) in randomized, double-blind, controlled clinical studies. Here, the efficacy and safety profile of fingolimod in Hispanic patients was compared to that observed in the overall study populations.
Methods
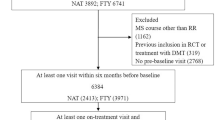
This was a post hoc analysis of relapses and safety data for Hispanic patients with relapsing–remitting MS (RRMS) randomized to receive daily fingolimod 0.5 mg, weekly IFNβ-1a IM (30 mg) or placebo, in the phase 3, controlled FREEDOMS, FREEDOMS II, and TRANSFORMS fingolimod studies. The ARR was estimated for each treatment group; only relapses that were confirmed by an independent examining neurologist were included in these analyses. Safety assessments included the incidence of adverse events and serious adverse events.
Results
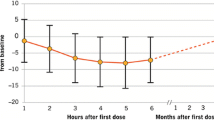
Eligible Hispanic patients aged 18–55 years (n = 181) had been treated as follows: fingolimod 0.5 mg (n = 89), IFNβ-1a IM (n = 65), and placebo (n = 27). Hispanic patients treated with fingolimod for up to 2 years had lower ARRs (ARR: 0.22, 95% confidence interval [CI]: 0.14–0.35) than those receiving placebo (ARR: 0.46, 95% CI: 0.24–0.88) or IFNβ-1a IM (ARR: 0.34, 95% CI: 0.18–0.63), with relative reductions of 52% and 35%, respectively. A transient decrease in heart rate that started to attenuate 6 h after fingolimod administration was observed, consistent with the well-characterized pharmacologic effect following fingolimod treatment initiation. No cases of symptomatic bradycardia were reported in Hispanic patients. The incidence of first-degree atrioventricular block was low and similar across all treatment groups (3.1–4.5%). The safety profile of fingolimod in Hispanic patients was consistent with that reported in the overall population of each study.
Conclusion
Overall, this study demonstrates that fingolimod is efficacious and well tolerated in Hispanic patients with RRMS.

Similar content being viewed by others
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–31.
Multiple Sclerosis International Federation. Atlas of MS. 2013. Available at: http://www.msif.org/includes/documents/cm_docs/2013/m/msif-atlas-of-ms-2013-report.pdf?f=1. Accessed on August 6, 2014.
Inglese M. Multiple sclerosis: new insights and trends. AJNR Am J Neuroradiol. 2006;27:954–7.
Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133–46.
Correale J. MS in Latin America. Int MS J. 2008;15:3–5.
Amezcua L, Lund BT, Weiner LP, et al. Multiple sclerosis in Hispanics: a study of clinical disease expression. Mult Scler. 2011;17:1010–6.
Corona T, Roman GC. Multiple sclerosis in Latin America. Neuroepidemiology. 2006;26:1–3.
US Food and Drug Administration. GILENYA® (fingolimod) prescribing information. 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022527s009lbl.pdf. Accessed on August 6, 2014.
Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–56.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401.
Chinea Martinez AR, Meng X, Randhawa S. Fingolimod first-dose effects in Hispanic patients with relapsing-remitting multiple sclerosis. CMSC Annual meeting 2014. Dallas, TX, USA, May 28–31, 2014.
Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Annal Neurol. 2005;58:840–6.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Schmouder R, Serra D, Wang Y, et al. FTY720: placebo-controlled study of the effect on cardiac rate and rhythm in healthy subjects. J Clin Pharmacol. 2006;46:895–904.
Gold R, Comi G, Palace J, et al. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol. 2014;261:267–76.
Acknowledgments
This study and article processing charges were funded by Novartis Pharmaceuticals Corporation. Oxford PharmaGenesis® Ltd provided editorial support during the development of this manuscript. Funding for this editorial support was provided by Novartis Pharmaceuticals Corporation. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Angel R Chinea Martinez is a consultant for and is a member of the speaker bureau for Allergan, Biogen Idec, Novartis, Sanofi-Genzyme, and Teva. He has also received grant/research support from Biogen Idec, Novartis, and Teva. Jorge Correale has served as an advisory board member for Merck Serono Argentina, Novartis Argentina, Biogen Idec, and Merck Serono. He has received reimbursement for developing educational presentations for Merck Serono Argentina, Biogen Idec Argentina, and TEVA Tuteur Argentina, as well as professional travel and accommodation stipends. Patrick K Coyle has received grant/research support or honoraria for educational or consultative services related to MS from the following companies: Acorda, Actelion, Avanir, Bayer, Biogen Idec, EMD Serono, Novartis, Questcor, Roche, Sanofi Aventis, and Teva Neurosciences. Xiangyi Meng is an employee of Novartis Pharmaceuticals Corporation. Nadia Tenenbaum is an employee of Novartis Pharmaceuticals Corporation.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
Funding
Sponsorship for this study was funded by Novartis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrials.gov # NCT00289978 (FREEDOMS), NCT00355134 (FREEDOMS II), NCT00340834 (TRANSFORMS).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chinea Martinez, A.R., Correale, J., Coyle, P.K. et al. Efficacy and Safety of Fingolimod in Hispanic Patients with Multiple Sclerosis: Pooled Clinical Trial Analyses. Adv Ther 31, 1072–1081 (2014). https://doi.org/10.1007/s12325-014-0154-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0154-4