Abstract
Background
The ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter) trial demonstrated a significant reduction (26%) in the rate of first cardiovascular (CV) hospitalization in dronedarone-treated patients with paroxysmal or persistent atrial fibrillation/flutter (AF/AFL). ATHENA was the first trial to demonstrate a CV outcomes benefit, specifically reduced CV hospitalizations, with an antiarrhythmic drug. The objective of this study was to assess the impact of dronedarone treatment on healthcare resource utilization among real-world patients with AF/AFL in United States clinical practice.
Methods
This retrospective cohort study used claims data from the MarketScan® databases (Truven Health, Durham, NC, USA) to identify patients with ≥2 concurrent de novo pharmacy claims for dronedarone (≥180 days’ total supply) between June 2009 and March 2011, and with an AF/AFL diagnosis and no heart failure-related hospitalization during the 12 months preceding the initial (index) dronedarone claim. Annualized inpatient and outpatient resource utilization were compared between the pre-index (baseline) and post-index (follow-up) periods.
Results
In total, 5,656 AF/AFL patients were prescribed dronedarone for ≥6 months and were followed for mean (standard deviation) 11.9 (4.7) months. Reductions in mean numbers of annualized all-cause, CV- and AF-related hospitalizations (~40–45%), and emergency department visits (~30–45%) were realized. These benefits were offset by increases in office visits (~10–30%) and AF-related prescription claims (74%) after dronedarone initiation. The sub-cohort of patients switching to dronedarone from Prior Rhythm-Control therapy (n = 2,080) showed similar reductions in hospital and emergency department events.
Conclusions
This study suggests that dronedarone use in real-world practice, as in the ATHENA trial, results in substantial reductions in hospital admissions, both in first-line and second-line antiarrhythmic treatment settings.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) and atrial flutter (AFL) collectively affect an estimated 3.3 million adults in the USA and confer increased cardiovascular and cerebrovascular risk [1]. Patients with AF/AFL are more likely than age-matched individuals without AF/AFL to have other cardiovascular and cardio-metabolic conditions [1–3]. Moreover, the presence of AF/AFL worsens the prognosis of patients with cardiovascular comorbidities, elevates the risk of stroke and doubles the risk of mortality [4, 5]. AF is considerably more frequent than AFL, and accounts for >90% of all cases of AF/AFL [1]. In the USA, AF-associated hospitalizations and deaths have been increasing steadily over the past 30 years [6], and one in three patients can expect a cardiovascular-related hospitalization within the first year following diagnosis of AF [6].
Clinical trials have provided evidence of significant improvements in patient-centered outcomes in AF, including decreased rates of stroke, cardiovascular hospitalization, and cardiovascular mortality [7–9]. Whether trial-tested outcomes translate into improved outcomes in the real-world setting is not well studied and remains an important clinical question. Although randomized clinical trials are important in establishing treatment efficacy, their emphasis on internal validity often limits the generalizability of their findings [10]. Differences in patient selection, treatment conditions, and practice patterns between the clinical trial and the real-world setting can influence treatment outcomes [11]. Important patient subgroups, including the elderly, and those with coexisting morbidities, are frequently under-represented in clinical trials of cardiovascular drugs [12, 13]. Likewise, AF clinical trial populations vary in their representativeness to real-world AF patients [14, 15].
Large, observational cohort studies can be useful in bridging the gap between the highly controlled environment of the randomized clinical trial and routine clinical practice. Observational studies can enroll more heterogeneous populations, including older patients and those with comorbidities, provide extended patient follow-up, and incorporate a diversity of outcome measures. Retrospective cohort studies have been helpful in determining the resource use and cost burden associated with disease management among real-world AF/AFL patients [8, 10, 16].
The recent ATHENA trial (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter; NCT 00174785), demonstrated significant reductions in cardiovascular-related hospitalization rates among dronedarone-treated patients with AF/AFL (26%) [9]. The objective of this retrospective, observational study was to assess the impact of dronedarone treatment on healthcare resource utilization among real-world patients with AF/AFL in USA clinical practice.
Methods
Data Source
This study used administrative claims data from the Thomson Reuters MarketScan® Commercial Claims and Encounter database and Medicare Supplemental and Coordination of Benefits database (Truven Health, Durham, NC, USA) for the period June 2008–September 2011. The Commercial Claims database contains health claims records from privately insured individuals and their dependents, whereas the Medicare Supplemental database focuses on the over 65-year-old population with employer-sponsored Medicare-supplemental healthcare insurance. Collectively, the databases capture patient-level healthcare claims data from more than 100 different insurance companies, Blue Cross Blue Shield plans, and third-party administrators. The claims data include inpatient and outpatient information, health and productivity data, laboratory data, and hospital drug data. The MarketScan® databases include 43.1 million patients across the continental USA, including 39.9 million commercial lives and 3.2 million Medicare lives with employer-sponsored supplemental insurance (2009 data) [17]. The databases are fully compliant with the Health Portability and Accountability Act of 1996 (HIPAA), and all data are de-identified to protect the privacy of patients and providers. Accordingly, institutional review board and ethics committee approval was not required for the study.
Patient Selection
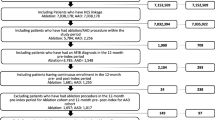
Patients were ≥18 years of age, with a diagnosis of AF and/or AFL (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 427.31 and 427.32, respectively) [18] on ≥1 inpatient claim or ≥2 outpatient claims on different days between June 2008 and September 2011, and received ≥2 prescriptions for dronedarone, amounting to ≥180 days’ continuous supply, on separate dates between June 2009 and March 2011. The first prescription claim during this period was identified as the index claim, and the date of this claim was the index date. Patients were required to have continuous medical and prescription drug insurance coverage for 12 months prior to the index date (baseline period) and for ≥6 months after the index date, until 30 days after the end of the dronedarone possession period or the end of the health plan enrollment, whichever occurred first (follow-up period). The dronedarone possession period was defined as the time from the index date to the end of the days-of-supply of the last dronedarone prescription, allowing a grace period of ≤30 days between exhaustion of drug supply from one prescription and fill of the next prescription. Patients were excluded if they had evidence of transient AF/AFL, as indicated by an ICD-9-CM diagnosis code for hyperthyroidism or a prescription for methimazole or propylthiouracil during the baseline period or an AF/AFL diagnosis within 30 days of cardiac surgery; two or more cardioversion procedures [Current Procedural Terminology, 4th edition (CPT-4) procedure codes 92960, 92961; ICD-9 procedure code 99.61] in the 3 months prior to the index date; or were hospitalized for heart failure during the baseline period.
Patients satisfying the study eligibility criteria comprised the full study population; a subset of patients who received a rhythm-control agent within 3 months prior to initiation of dronedarone was identified (“Prior Rhythm-Control cohort”).
Outcome Measures
Patient demographics were recorded at the time of the index prescription claim. Clinical characteristics, including comorbidities (defined according to ICD-9-CM diagnosis codes), Charlson Comorbidity Index (CCI) score [19] and CHADS2 [Congestive heart failure, history of Hypertension, Age ≥75 years, Diabetes mellitus, and past history of Stroke or TIA (transient ischemic attack)] score [20], and AF-related medication (rhythm-control, rate-control, and anticoagulation) use were determined over the baseline period.
Healthcare resource utilization, comprising inpatient services (hospital facility and professional), outpatient medical services (emergency department and office visits, laboratory tests and procedures), and outpatient pharmacy services, were determined over the baseline and follow-up periods. Inpatient and outpatient medical services were stratified by cause (all-cause, cardiovascular-related, and AF-related), and outpatient pharmacy services were stratified by treatment type (all-cause and AF-related). To control for the variable duration of post-index follow-up, resource utilization was calculated on an annual basis. Office and emergency department visits were defined as the number of days with an outpatient claim, as the MarketScan® databases do not identify each individual visit. Resource utilization was expressed as the number of hospital admissions and total number of days in hospital, the number of days with office and emergency department visits, and the proportion of patients with prescription drug claims per patient-year (PPY) for each study period. Intra-cohort comparisons of annualized healthcare resource utilization between baseline and follow-up periods were conducted for (1) the full study population and (2) the Prior Rhythm-Control cohort.
Statistical Analysis
Hospitalization rates, resource utilization, and costs were calculated on an annual basis, as follows: annualized outcome = outcome/number of days in observation period × 365. The baseline period was of uniform duration (365 days) for all patients. The post-index follow-up period varied from patient to patient. Bivariate statistics were used to compare healthcare resource utilization between the baseline and follow-up periods (intra-cohort comparison). Student’s paired t test was used for comparison of continuous variables with a 5% significance level (α = 0.05). All statistical analyses were carried out using SAS Release 9.2 (SAS Institute, Inc., Cary, NC, USA).
The analysis in this article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Patient Characteristics
In total, 5,656 AF/AFL patients met the study eligibility criteria (full study population) and were prescribed dronedarone for ≥6 months. The full study population was predominantly male (62%), had a mean age of 68.3 years, and included patients enrolled in managed care systems as well as fee-for-service health plans (Table 1). A high proportion of patients had comorbidities, most commonly hypertension (66.8%), coronary artery disease (35.5%), and diabetes (22.7%). During the baseline period, most patients received rate-control (β-blocker and calcium channel blocker) therapy (80.5%) and anticoagulant (predominantly warfarin) therapy (56.3%); less than one-half of patients received rhythm-control (predominantly amiodarone) therapy (41.3%). The mean [standard deviation (SD)] duration of post-index follow-up was 11.9 (4.7) months (range 6.0–26.1 months).
Of the 5,656 patients in the full study population, 2,080 patients had received rhythm-control treatment in the 3 months prior to the index dronedarone prescription claim (Prior Rhythm-Control cohort). The demographic and baseline clinical characteristics of this cohort closely mirrored those of the full study population (Table 1), with the exception of baseline AF-related therapy, which for the Prior Rhythm-Control cohort comprised rhythm-control therapy (100%), rate-control therapy (80.3%), and anticoagulation (67.1%). The mean (SD) duration of follow-up for the Prior Rhythm-Control cohort was 12.4 (5.0) months (range 6.0–25.6 months).
Healthcare Resource Utilization
Full Study Population
Mean numbers of annualized all-cause, cardiovascular- and AF-related hospitalizations, and associated lengths of hospital stay were significantly (P < 0.0001) reduced from baseline levels following initiation of dronedarone treatment (Fig. 1). Significant reductions were noted in the numbers of all-cause hospitalizations (−39%) and emergency department visits (−27%) and in the total length of hospital stay (−35%) (all P < 0.0001); in contrast, there were significant increases in the number of all-cause office visit claims (+7%) and outpatient prescription claims (+24%) (both P < 0.0001) after dronedarone initiation (Fig. 2). Similar reductions were noted in mean numbers of cardiovascular-related and AF-related hospitalizations (−42% and −46% respectively, P < 0.0001) and cardiovascular-related and AF-related emergency department visits (−41% and −45% respectively, P < 0.0001), and in total lengths of cardiovascular-related and AF-related hospital stay (−37% and −42%, P < 0.0001). The increases in mean numbers of cardiovascular- and AF-related office visits (+12% and +29%, P < 0.0001) and AF-related prescription claims (+74%, P < 0.0001) (Figs. 1, 2) were offset by the reductions in hospitalizations and emergency department visits.
Comparison of AF-related prescription drug utilization before and after initiation of dronedarone indicated high rates of uptake of rate-control therapy during both study periods (80.5% vs. 78.1%). Use of rhythm-control agents other than dronedarone declined between the baseline and follow-up period (41.3% vs. 11.8%), whereas anticoagulant coverage increased (56.3% vs. 68.5%). Mean numbers of antiarrhythmic drug claims increased almost fourfold from baseline levels following initiation of dronedarone treatment (7.5 vs. 1.9 claims PPY) (P < 0.0001). This increase was almost entirely due to claims for dronedarone itself (mean 7.3 claims PPY), since the follow-up period was marked by large percent reductions in mean numbers of prescription claims for amiodarone (−85%), sotalol (−91%), and other antiarrhythmic drugs (–89%) (all P < 0.0001) (Fig. 3). Initiation of dronedarone treatment was also associated with increases in the mean number of prescription claims for anticoagulant drugs (+65%), including warfarin (+46%) and dabigatran (+2,400%) (all P < 0.0001). In contrast, numbers of prescription claims for rate-control agents, including calcium channel blockers and β-blockers, remained largely unchanged after the introduction of dronedarone (Table 2).
Prior Rhythm-Control Cohort
Similar changes in pattern of healthcare resource utilization to those described in the full study population occurred in the Prior Rhythm-Control cohort after initiation of dronedarone (Figs. 1, 2). Marked reductions in the mean numbers of all-cause hospitalizations (−33%) and emergency department visits (−36%) and in the total length of hospital stay (−34%) were accompanied by an increase in the mean number of all-cause prescription claims (+13%) (all P < 0.0001) after introduction of dronedarone (Figs. 1, 2). Cardiovascular- and AF-related hospitalizations (−35% and −39%), emergency department visits (−49% and −53%), and AF-related prescription claims (+26%), as well as durations of cardiovascular- and AF-related hospital stay (−36% and −37%) (all P < 0.0001) were affected in a similar manner (Figs. 1, 2).
Comparison of treatment patterns between the baseline and follow-up periods revealed similar levels of coverage with rate-control therapy (80.3% vs. 77.5%) and anticoagulant therapy (67.1% vs. 69.1%), but reduced use of other rhythm-control agents (18.0%) after introduction of dronedarone. Despite sharp reductions (≥90%) in numbers of claims for amiodarone and sotalol, the overall mean number of antiarrhythmic drug claims during the follow-up period was 55% higher than during the baseline period, with dronedarone accounting for most of these (Table 2). Initiation of dronedarone treatment was also associated with increases in mean numbers of prescription claims for anticoagulant drugs (+22%, P < 0.0001), including warfarin (+10%, P < 0.001) and dabigatran (+2,000%, P < 0.0001) (Table 2).
Discussion
The present analysis reinforces the findings of the ATHENA trial by indicating that the reductions in cardiovascular hospitalization obtained with dronedarone in the controlled clinical trial setting extend to the broader population of AF/AFL patients—both those initiating rhythm-control therapy and those switching from other rhythm-control agents—in real-world practice. These data are of value in that the ATHENA trial population, which excluded patients with severe heart failure and permanent AF, was similar to the current study population. The cardiovascular outcome benefit of reduced cardiovascular hospitalizations seen in the real world helps to validate both the clinical trial findings from ATHENA and the recommendations of the American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society (ACCF/AHA/HRS) AF-focused guideline update regarding dronedarone [21].
The ATHENA trial population comprised patients with paroxysmal or persistent AF/AFL who were aged either ≥75 years (with or without cardiovascular risk factors) or <75 years with one or more additional cardiovascular risk factors. Patients with recently decompensated heart failure or severe heart failure [New York Heart Association (NYHA) class IV] were excluded. Over a mean follow-up period of 21 months, dronedarone reduced (inter alia) the risk of first cardiovascular hospitalization by 26% relative to placebo [9].
On the basis of the ATHENA trial findings, dronedarone was initially approved by the US Food and Drug Administration (FDA) in 2009 to reduce the risk of cardiovascular hospitalization in patients with paroxysmal or persistent AF/AFL, with a recent episode of AF/AFL and associated cardiovascular risk factors, and in sinus rhythm or scheduled for cardioversion. A subsequent FDA review, prompted by the PALLAS (Permanent Atrial fibriLLation Outcome Study Using Dronedarone on Top of Standard Therapy) trial findings [22], led to the revised recommendation that dronedarone should be initiated only in patients with non-permanent AF (but not AFL) who are in sinus rhythm, and discontinued on progression to permanent AF [23]. The therapeutic value of dronedarone, including its risks and benefits, has been reviewed extensively in several articles [24–26], with the general consensus that patients being considered for dronedarone therapy be carefully selected and monitored to prevent potential adverse events and drug interactions. As such, current 2011 American College of Cardiology/American Heart Association (ACC/AHA) practice guidelines for management of AF recommend dronedarone as a treatment that can be initiated in the outpatient setting to reduce the need for cardiovascular hospitalization in patients with paroxysmal AF or after cardioversion of persistent AF [21, 27].
The study population, drawn from patients who initiated dronedarone treatment between June 2009 and March 2011 following a recent diagnosis of AF/AFL, is likely to have satisfied the clinical criteria of the initial (pre-2011) product label, and appears to have been at comparable cardiovascular risk to the ATHENA trial population. By excluding patients with recent hospitalization for heart failure, it is likely that any patients with significant heart failure were removed from the study. Patients had a high level of baseline cardiovascular/cardio-metabolic comorbidity, most commonly hypertension, coronary artery disease, and diabetes, and were of similar age (mean 68.4 years) to the ATHENA trial population (mean 71.6 years). Patients were required to have a minimum of 180 days of continuous use of dronedarone both to ensure treatment compliance and to match the treatment duration of the ATHENA trial. Results from this real-world population indicated that initiation of dronedarone treatment resulted in pronounced reductions in all-cause, cardiovascular-related, and AF-related hospital admissions (~40–45%), emergency department visits (~30–45%), and lengths of hospital stay (~35–40%), which were offset by modest increases in office visits (~10–30%) and a marked increase in AF-related drug (predominantly dronedarone) prescriptions (74%) over the following 12 months. Numerically similar changes in healthcare resource utilization to those in the full study population occurred in the Prior Rhythm-Control cohort over this period; reductions in all-cause, cardiovascular-related, and AF-related hospital admissions, emergency department visits, and lengths of hospital stay were accompanied by an increase in AF-related prescriptions.
In clinical practice, AF/AFL patients at high thromboembolic risk, who represent prime candidates for dronedarone treatment, experience high rates of hospitalization, with approximately 40% requiring readmission within the first 12 months after their initial hospitalization for AF/AFL, resulting in a mean cost per AF hospitalization of US$7,476–8,493 [16, 28]. In keeping with the high volume and temporal pattern of cardiovascular admissions displayed by this high-risk patient population, the study findings suggest that the benefit of reduced hospital resource utilization associated with dronedarone is realized promptly, within 12 months of commencing treatment.
With the healthcare costs of AF/AFL currently estimated at $26 billion annually [29], and the number of cases of AF/AFL in the US predicted to rise to more than 8 million over the next 30–40 years [1], the development of treatments that can reduce the need for cardiovascular admission assumes importance in restraining the growing cost burden of this condition. The recent ACC and Institute for Healthcare Improvement-led Hospital to Home initiative was designed to reduce 30-day all-cause readmission rates for patients discharged with heart failure or acute myocardial infarction by 20% nationwide [30]. It is feasible that a national quality initiative of this type might also be applied to the management of AF/AFL, with the aim of reducing hospital admissions and improving the transition of care from the inpatient to outpatient setting; one element of this would be improvement of post-discharge medication management.
Limitations of this study include its retrospective, nonrandomized design, the absence of a control group, meaning a placebo effect cannot be eliminated, and reliance on data from a highly regulated, US-specific, healthcare system. Use of the MarketScan® databases has the advantage of capturing the reimbursement policies and practices of multiple payers; however, the applicability of the study findings to Medicare patients without employer-sponsored supplemental insurance and to the uninsured is uncertain. The MarketScan® databases are also limited to the diagnostic information that supports claims for reimbursement, and errors may arise through coding inaccuracies. Furthermore, there was little information on the causality and severity of AF/AFL, and the time interval from disease diagnosis to initiation of dronedarone therapy was variable. Given the constraints of the administrative claims data and the non-randomized design of the study, it was not possible to assess the contributions of patient profile, the AF disease process, and rhythm- or rate-control therapy to the observed clinical outcomes. It is unclear whether the patients switching from Prior Rhythm-Control therapy were doing so because of treatment failure or on account of the more favorable tolerability profile of dronedarone. Although intra-cohort comparison of baseline versus follow-up outcomes allowed patients to serve as their own controls, the absence of a control group prevented assessment of the potential influence of disease evolution on the measured outcomes.
In the study, patients were required to have received dronedarone for at least 180 days. Thus, the patients receiving a shorter duration of dronedarone treatment were not evaluated. Also, the study did not take into consideration the monitoring requirements for dronedarone introduced in the post-approval period. The small increase in office visits observed with patients receiving dronedarone was not controlled for, and may have an impact on the numbers of hospitalizations and emergency department events. The use of anticoagulation during the 12-month baseline period appears to be higher in the Prior Rhythm-Control cohort than in the full study population (67.1% vs. 56.3% of patients). As a potential hypothesis, the patients in the Prior Rhythm-Control cohort may have been considered to have more severe AF/AFL and/or at a higher risk of stroke. Similarly, the increase in anticoagulation use following dronedarone initiation (coverage rose to ~69% of patients in both groups) may have been a consequence of the increased number of office visits (i.e., improved patient care) and closer adherence to treatment guidelines. Thus, it cannot be excluded that some of the benefits associated with dronedarone may be due to improved anticoagulation and/or overall patient care. Finally, patients selected for inclusion in this study were required to have demonstrated reasonable persistence (≥6 months) with dronedarone therapy, in keeping with the ATHENA trial population, whereas in clinical practice initial rhythm-control therapy is often associated with high rates of discontinuation [31].
Conclusions
This study extends the findings of the ATHENA trial by indicating that either first- or second-line treatment with dronedarone substantially reduces the rate of cardiovascular hospitalization among AF/AFL patients in real-world practice. The reduction in number and duration of hospitalizations that follows initiation of dronedarone treatment is offset in part by a modest increase in office visits and a more marked increase in AF-related prescriptions claims (mainly on account of claims for dronedarone itself).
References
Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–9.
Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–84.
Estes NA 3rd, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, et al. ACC/AHA/Physician Consortium 2008 Clinical Performance Measures for Adults with Nonvalvular Atrial Fibrillation or Atrial Flutter: a report of the American College of Cardiology/American Heart Association Task Force on performance measures and the physician consortium for performance improvement (writing committee to develop clinical performance measures for atrial fibrillation) developed in collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2008;51:865–84.
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52.
Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–64.
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Seward JB, et al. Changing trends of hospital utilization in patients after their first episode of atrial fibrillation. Am J Cardiol. 2008;102:568–72.
Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–78.
Freemantle N, Strack T. Real-world effectiveness of new medicines should be evaluated by appropriately designed clinical trials. J Clin Epidemiol. 2010;63:1053–8.
Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials. 2009;10:37.
Wieringa NF, de Graeff PA, van der Werf GT, Vos R. Cardiovascular drugs: discrepancies in demographics between pre- and post-registration use. Eur J Clin Pharmacol. 1999;55:537–44.
Wieringa NF, Vos R, van der Werf GT, van der Veen WJ, de Graeff PA. Co-morbidity of ‘clinical trial’ versus ‘real-world’ patients using cardiovascular drugs. Pharmacoepidemiol Drug Saf. 2000;9:569–79.
Lakdawalla D, Turakhia MP, Jhaveri M, Mozaffari E, Davis P, Bradley L, et al. Comparative effectiveness of antiarrhythmic drugs on cardiovascular hospitalization and mortality in atrial fibrillation. J Comp Eff Res. 2013;2:301–12.
Lee S, Monz BU, Clemens A, Brueckmann M, Lip GY. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: a cross-sectional analysis using the General Practice Research Database. BMJ Open. 2012;2(pii: e001768).
Naccarelli GV, Johnston SS, Lin J, Patel PP, Schulman KL. Cost burden of cardiovascular hospitalization and mortality in ATHENA-like patients with atrial fibrillation/atrial flutter in the United States. Clin Cardiol. 2010;33:270–9.
Hansen LG, Chang S. Health research data for the real world: the MarketScan databases. MarketScan White Paper 2012. http://www.truvenhealth.com/assets/2012_Truven_MarketScan_white_paper.pdf. Accessed 8 Jan 2014.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed 8 Jan 2014.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70.
Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA 3rd, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–42.
Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–76.
U.S. Food and Drug Administration. Multaq (dronedarone): Drug Safety Communication—Increased Risk of Death or Serious Cardiovascular Events. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm264204.htm. Accessed Sept 24 2013.
Heijman J, Heusch G, Dobrev D. Pleiotropic effects of antiarrhythmic agents: dronedarone in the treatment of atrial fibrillation. Clin Med Insights Cardiol. 2013;7:127–40.
Naccarelli GV, Wolbrette DL, Levin V, Samii S, Banchs JE, Penny-Peterson E, Gonzalez MD. Safety and efficacy of dronedarone in the treatment of atrial fibrillation/flutter. Clin Med Insights Cardiol. 2011;5:103–19.
Patel PD, Bhuriya R, Patel DD, Arora BL, Singh PP, Arora RR. Dronedarone for atrial fibrillation: a new therapeutic agent. Vasc Health Risk Manag. 2009;5:635–42.
Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS guideline recommendations: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:1935–44.
Amin A, Jhaveri M, Lin J. Hospital readmissions in US atrial fibrillation patients: occurrence and costs. Am J Ther. 2013;20:143–50.
Kim MH, Johnston SS, Chu B-C, Dalal MR, Schulman KS. Estimation of total incremental health care cost in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–20.
American College of Cardiology, Institute for Healthcare Improvement. Hospital to Home, Reducing Readmissions, Improving Transitions (H2H National Quality Improvement Initiative). http://www.h2hquality.org. Accessed 24 Sept 2013.
Kim MH, Klingman D, Lin J, Battleman DS. Patterns and predictors of discontinuation of rhythm-control drug therapy in patients with newly diagnosed atrial fibrillation. Pharmacotherapy. 2009;29:1417–26.
Acknowledgments
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Sponsorship and article processing charges for this study were funded by sanofi-aventis US Inc. (Bridgewater, NJ, USA). Medical writing assistance for this study was provided by Andrew Fitton, PhD, of Excel Scientific and was funded by sanofi-aventis US. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Dr Kim is a consultant to sanofi-aventis US, Inc. Dr Lin is a director of Novosys Health, which has a research consultancy agreement with sanofi-aventis US, Inc. Dr Jhaveri is an employee of sanofi-aventis US, Inc. Dr Koren is an employee of sanofi-aventis US, Inc.
Compliance with ethics guidelines
The databases used in this study are fully compliant with the Health Portability and Accountability Act of 1996 (HIPAA), and all data are de-identified to protect the privacy of patients and providers. Accordingly, institutional review board and ethics committee approval was not required for the study. The analysis in this article is based on retrospectively collected data, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kim, M.H., Lin, J., Jhaveri, M. et al. Impact of Dronedarone Treatment on Healthcare Resource Utilization in Patients with Atrial Fibrillation/Flutter. Adv Ther 31, 318–332 (2014). https://doi.org/10.1007/s12325-014-0108-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-014-0108-x