Abstract
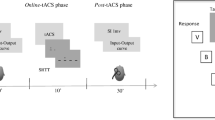
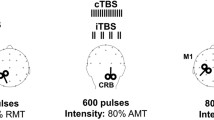
Converging evidence suggests a crucial role of right inferior frontal gyrus (r-IFG) and right pre-supplementary motor area (r-preSMA) in movement inhibition control. The present work was aimed to investigate how the effective connectivity between these prefrontal areas and the primary motor cortex could change depending on the activity of the cerebellar cortex. Paired transcranial magnetic stimulation (TMS) was delivered in healthy subjects over the r-IFG/left primary motor area (l-M1) and over r-preSMA/l-M1 before (100 ms after the fixation cross onset) and 50, 75, 100, 125, and 150 ms after the presentation of a Go/NoGo visual cue establishing the specific time course and the causal interactions of these regions in relation to l-M1 as measured by motor evoked potentials (MEPs). The same paired-pulse protocol was applied following sham or real cerebellar continuous theta burst stimulation (cTBS). Following sham cTBS, for NoGo trials only, MEPs collected showed the expected pattern of activation for both r-IFG-l-M1 and r-preSMA-l-M1 connectivity, characterized by peaks of increased and decreased MEP amplitude regularly repeated every 50 ms. Following cerebellar cTBS, this pattern of activation related to NoGo trials was modified selectively for the r-IFG-M1 but not for r-preSMA-M1 connection. A common monitoring action of r-IFG and r-preSMA in inhibitory control was confirmed. The effects of cerebellar cTBS showed a specific interaction between cerebellum and r-IFG activity during the inhibitory process.



Similar content being viewed by others
References
Obeso I, Wilkinson L, Casabona E, Bringas ML, Álvarez M, Álvarez L, et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp Brain Res 2011; doi: 10.1007/s00221-011-2736-6
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–52.
Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 2009; doi:10.1523/JNEUROSCI.1300-09.2009.
Tabu H, Mima T, Aso T, Takahashi R, Fukuyama H. Common inhibitory prefrontal activation during inhibition of hand and foot responses. Neuroimage 2012; doi: 10.1016/j.neuroimage.2011.10.092
Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, et al. Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol 2014; doi:10.1016/j.cub.2014.10.043
Picazio S, Koch G. Is Motor Inhibition Mediated by Cerebello-Cortical Interactions? Cerebellum 2015; doi:10.1007/s12311-014-0609-9.
Tanaka H, Harada M, Arai M, Hirata K. Cognitive dysfunction in cortical cerebellar atrophy correlates with impairment of the inhibitory system. Neuropsychobiology. 2003;47:206–11.
Smith AM, Walker LA, Freedman MS, DeMeulemeester C, Hogan MJ, Cameron I. fMRI investigation of disinhibition in cognitively impaired patients with multiple sclerosis. J Neurol Sci 2009; doi:10.1016/j.jns.2009.02.366
Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–22.
Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, et al. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology 2014; doi:10.1016/j.neuropharm.2013.05.027
Rubia K, Smith AB, Taylor E, Brammer M. Linear age correlated functional development of right inferior frontostriato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–77.
Hirose S, Jimura K, Kunimatsu A, Abe O, Ohtomo K, Miyashita Y, et al. Changes in cerebro-cerebellar interaction during response inhibition after performance improvement. Neuroimage 2014; doi:10.1016/j.neuroimage.2014.05.007
Picazio S, Oliveri M, Koch G, Caltagirone C, Petrosini L. Cerebellar contribution to mental rotation: a cTBS study. Cerebellum 2013; doi: 10.1007/s12311-013-0494-7
Daly E, Ecker C, Hallahan B, Deeley Q, Craig M, Murphy C, et al. Response inhibition and serotonin in autism: a functional MRI study using acute tryptophan depletion. Brain 2014; doi:10.1093/brain/awu178
Koch G, Mori F, Marconi B, Codecà C, Pecchioli C, Salerno S, et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol 2008. doi:10.1016/j.clinph.2008.08.008.
Pandey AK, Kamarajan C, Tang Y, Chorlian DB, Roopesh BN, Manz N, et al. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol 2012; doi:10.1016/j.biopsycho.2011.10.009.
Baümer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, et al. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest—a bifocal TMS study. Clin Neurophysiol. 2009;120:1724–31.
Ugawa Y, Lu MK, Bliem B, Murakami T, MullerDahlhaus F, Arai N, et al. State-dependent and timing- dependent bidirectional associative plasticity in the human SMA-M1 network. J Neurosc. 2011;31:15376–83.
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92.
Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, et al. In vivo definition of parieto-motor connections involved in planning of grasping movements. NeuroImage. 2010;51:300–12.
Koch G, Ponzo V, Di Lorenzo F, Caltagirone C, Veniero D. Hebbian and anti-Hebbian spike-timing-dependent plasticity of human cortico-cortical connections. J Neurosci. 2013;33:9725–33.
Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 2008; doi:10.1038/nrn2478
Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. J Neurophysiol. 2007;98:145–52.
Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–22.
Koch G, Brusa L, Carrillo F, Lo Gerfo E, Torriero S, Oliveri M, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology 2009; doi:10.1212/WNL.0b013e3181ad5387.
Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974;54:957–1006.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 2013; doi:10.1016/j.tics.2013.03.003.
Lamar M, Cutter WJ, Rubia K, Brammer M, Daly EM, Craig MC, et al. 5-HT, prefrontal function and aging: fMRI of inhibition and acute tryptophan depletion. Neurobiol Aging. 2009;30:1135–46.
Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci 2014; doi: 10.1523/JNEUROSCI.1776-14.2014.
Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res Cogn Brain Res. 2003;17:419–30.
Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–92.
van der Salm SM, van der Meer JN, Nederveen AJ, Veltman DJ, van Rootselaar AF, Tijssen MA. Functional MRI study of response inhibition in myoclonus dystonia. Exp Neurol. 2013; doi: 10.1016/j.expneurol.2013.02.017.
Obeso I, Robles N, Marrón EM, Redolar-Ripoll D. Dissociating the Role of the pre-SMA in Response Inhibition and Switching: A Combined Online and Offline TMS Approach. Front Hum Neurosci 2013; doi: 10.3389/fnhum.2013.00150
Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–61.
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum 2014; doi: 10.1007/s12311-013-0511-x
Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci 2005;25:7771–9
Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol 2009; doi:10.1016/j.cub.2009.07.074
Joundi RA, Jenkinson N, Brittain JS, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol 2012; doi: 10.1016/j.cub.2012.01.024
Acknowledgments
This work was supported by a grant from Italian Ministry of Health to G.K. (239/BR-2009-1591859).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a conflict of interest with respect to the purposes of the present study.
Rights and permissions
About this article
Cite this article
Picazio, S., Ponzo, V. & Koch, G. Cerebellar Control on Prefrontal-Motor Connectivity During Movement Inhibition. Cerebellum 15, 680–687 (2016). https://doi.org/10.1007/s12311-015-0731-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0731-3