Abstract
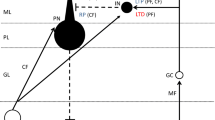
Long-term depression (LTD) at excitatory synapses between parallel fibers and a Purkinje cell has been regarded as a critical cellular mechanism for motor learning. However, it was demonstrated that normal motor learning occurs under LTD suppression, suggesting that cerebellar plasticity mechanisms other than LTD also contribute to motor learning. One candidate for such plasticity is rebound potentiation (RP), which is long-term potentiation at inhibitory synapses between a stellate cell and a Purkinje cell. Both LTD and RP are induced by the increase in postsynaptic Ca2+ concentration, and work to suppress the activity of a Purkinje cell. Thus, LTD and RP might work synergistically, and one might compensate defects of the other. RP induction is dependent on the interaction between GABAA receptor and GABAA receptor binding protein (GABARAP). Transgenic mice expressing a peptide which inhibits binding of GABARAP and GABAA receptor only in Purkinje cells show defects in both RP and adaptation of vestibulo-ocular reflex (VOR), a motor learning paradigm. However, another example of motor learning, adaptation of optokinetic response (OKR), is normal in the transgenic mice. Both VOR and OKR are reflex eye movements suppressing the slip of visual image on the retina during head movement. Previously, we reported that delphilin knockout mice show facilitated LTD induction and enhanced OKR adaptation, but we recently found that VOR adaptation was not enhanced in the knockout mice. These results together suggest that animals might use LTD and RP differently depending on motor learning tasks.
Similar content being viewed by others
References
Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–95.
Hirano T. Long-term depression and other synaptic plasticity in the cerebellum. Proc Jpn Acad B. 2013;89:183–95.
Welsh JP, Yamaguchi H, Zeng XH, Kojo M, Nakada Y, Takagi A, et al. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc Natl Acad Sci U S A. 2005;102:17166–71.
Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50.
Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43.
Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35.
Ohtsuki G, Piochon C, Adelman JP, Hansel C. SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron. 2012;75:108–20.
Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–4.
Tanaka K, Khiroug L, Santamaria F, Doi T, Ogasawara H, Ellis-Davies G, et al. Ca2+ requirements for cerebellar long-term synaptic depression: role for a postsynaptic leaky integrator. Neuron. 2007;54:787–800.
Kitagawa Y, Hirano T, Kawaguchi S. Prediction and validation of a mechanism to control the threshold for inhibitory synaptic plasticity. Mol Syst Biol. 2009;5(280):1–16.
Kawaguchi S, Nagasaki N, Hirano T. Dynamic impact of temporal context of Ca2+ signals on inhibitory synaptic plasticity. Sci Rep. 2011;1(143):1–12.
Kawaguchi S, Hirano T. Gating of long-term depression by CaMKII through enhanced cGMP signaling in cerebellar Purkinje cells. J Physiol. 2013;591:1707–30.
Hirano T, Kawaguchi S. Regulation and functional roles of rebound potentiation at cerebellar stellate cell-Purkinje cell synapse. Front Cell Neurosci. 2014;8(42):1–8.
Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;7:377–88.
Kawaguchi S, Hirano T. Suppression of inhibitory synaptic potentiation by presynaptic activity through postsynaptic GABAB receptors in a Purkinje neuron. Neuron. 2000;27:339–47.
Kawaguchi S, Hirano T. Sustained GABARAP structural change underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–99.
Tanaka S, Kawaguchi S, Shioi G, Hirano T. Long-term potentiation of inhibitory synaptic transmission onto cerebellar Purkinje neurons contributes to adaptation of vestibulo-ocular reflex. J Neurosci. 2013;33:17209–20.
Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, et al. Disturbed motor coordination, Purkinje cell synapse formation and cerebellar long-term depression of mice defective in the δ2 subunit of the glutamate receptor channel. Cell. 1995;81:245–52.
Hirano T. Cerebellar regulation mechanisms learned from studies on GluRδ2, a unique glutamate-receptor-related molecule specifically expressed at parallel fiber-Purkinje cell synapses. Mol Neurobiol. 2006;33:1–16.
Takeuchi T, Ohtsuki G, Yoshida T, Fukaya M, Wainai T, Yamashita M, et al. Enhancement of both long-term depression induction and optokinetic response adaptation in mice lacking delphilin. PLoS One. 2008;3:e22 S97. 1–11.
Acknowledgments
We thank Drs. G. Ohtsuki and K. Funabiki for comments on the manuscript. This research was supported by a grant-in-aid for scientific research 25115716 in Japan to T. Hirano.
Conflict of Interest
The authors declare that they have no competing of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
7th Symposium of SRC
Rights and permissions
About this article
Cite this article
Hirano, T., Yamazaki, Y. & Nakamura, Y. LTD, RP, and Motor Learning. Cerebellum 15, 51–53 (2016). https://doi.org/10.1007/s12311-015-0698-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0698-0