Abstract
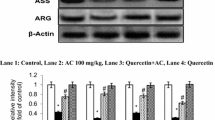
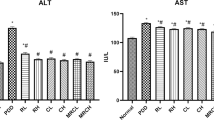
We have studied the ability of quercetin (a bioflavonoid) in tackling oxidative stress to alleviate the symptoms during ammonium chloride-induced hyperammonemia. Hyperammonemia was induced by the treatment of ammonium chloride (AC) 100 mg/kg b.w for 56 days. Hyperammonemic rats exhibited reduced urea (in plasma) and increased ammonia (in blood), uric acid (in plasma), creatinine (in serum), oxidative stress markers (thiobarbituric acid reactive substances (TBARS) and hydroperoxides (HP) and decreased levels of antioxidants (enzymatic and non-enzymatic) superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH) in plasma and tissues (liver and brain) vitamins E and C (in plasma)). The expression of liver inflammatory markers such as, interleukin 6 (IL-6), inducible nitric oxide synthase (iNOS) and nuclear transcription factor-κB (NF-κB) (by western blotting) were investigated. Histological damages (in liver, brain and kidney) were observed under hyperammonemia and the administration of quercetin (1) normalized the histopathological alterations, (2) reduced the levels of TBARS and HP, (3) elevated the antioxidants (SOD, CAT, GPx, GSH, vitamins E and C), (4) declined the activities of liver marker enzymes (AST, ALT and ALP) and (5) down regulated the expression of IL-6, iNOS and NF-κB. Our results suggest that quercetin might exert defense to AC-induced hyperammonemic rats to tackle (1) oxidative stress and (2) inflammation owing to its antioxidant, anti-inflammatory and cytoprotective effects.







Similar content being viewed by others
References
Jayakumar AR, Sujatha R, Paul V. Effect of ammonia on motor function in adult rats. Brain Res Bull. 1997;43:275–8.
Monfort P, Felipo V. Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase and cGMP-degrading phosphodiesterase, alterations in hyperammonemia. BMC Pharmacol. 2005;5:P66.
Majeed KI. Hyperammonemia is associated with an increase in inhibitory neurotransmission as a consequence of two factors. E Med J. 2005;2:2–15.
Wang C, Zhang SH, Wang PF, Li W, Lu J. Effects of ammonium on the antioxidative response in Hydrilla verticillata Royle plants. Ecotoxicol Environ Saf. 2010;73:189–95.
Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10.
Echeverry C, Arredondo F, Abin-Carriquiry JA, Midiwo JO, Ochieng C, Kerubo L. Pretreatment with natural flavones and neuronal cell survival after oxidative stress: a structure-activity relationship study. J Agric Food Chem. 2010;58:2111–5.
Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, et al. Neuroprotective effects of antioxidative flavonoids, quercetin, (1)—dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntiaficus-indica var. saboten. Brain Res. 2003;965:130–1.
Subramanian P, Jayakumar M, Jayapalan JJ, Hashim OH. Chronotherapeutic effect of fisetin on expression of urea cycle enzymes and inflammatory markers in hyperammonaemic rats. Pharmacol Rep. 2014;66:1037–42.
Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med. 2011;51:1329–36.
Wolheim DF. Preanalytical increase of ammonia in blood specimens from healthy subjects. Clin Chem. 1984;30:906–8.
Varley H, Gowenlock AH, Bell M. Practical clinical biochemistry, vol. 1. 4th ed. New Delhi: CBS Publishers; 1984.
Yagi K. Lipid peroxides and human disease. Chem Phys Lipids. 1987;45:337–51.
Fraga CG, Leibovitz BF, Toppel AL. Lipid peroxidation measured as TBARS in tissue slices. Characterization and comparison with homogenate and microsomes. Free Radic Biol Med. 1988;4:155–61.
Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low-density lipoprotein. Anal Biochem. 1992;202:384–9.
Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of SOD. Indian J Biochem Biophys. 1984;21:130–2.
Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94.
Ellman GC. Tissue sulfhydyl groups. Arch Biochem Biophys. 1959;82:70–7.
Rotruck JT, Pope AL, Ganther HE, Swason AB. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90.
Dimitrov NV, Meyer C, Gilliland D, Ruppenthal M, Chenoweth W, Malone W. Plasma tocopherol concentrations in response to supplemental vitamin E. Am J Clin Nutr. 1991;53:723–9.
Roe JH, Kuether CA. Detection of ascorbic acid in whole blood and urine through the 2,4-dinitrophyenyl hydrazine of dehydroascorbic acid. J Biol Chem. 1943;147:399–407.
Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta. 2003;327:69–79.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin Phenol reagent. J Biol Chem. 1951;193:265–75.
Jayakumar M, Subramanian P. Antihyperammonemic effect of fisetin on hyperammonemic rats: a biochemical study. Int J Mod Res Rev. 2013;2:33–9.
Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, et al. Inactivation of glutathione peroxidase by nitric oxide. Implication for cytotoxicity. J Biol Chem. 1995;270:21035–9.
Kosenko E, Kaminsky Y, Lopata O, Muravyov N, Kaminsky A, Hermenegildo C, et al. Nitroarginine, an inhibitor of nitric oxide synthase, prevents changes in superoxide radical and antioxidant enzymes induced by ammonia intoxication. Metab Brain Dis. 1998;13:29–41.
Kosenko E, Kaminsky Y, Grau E, Minana MD, Marcaida G, Grisolia S, et al. Brain ATP depletion induced by acute ammonia intoxication in rats is mediated by activation of the NMDA receptor and Na 1, K1-ATPase. J Neurochem. 1994;63:2172–8.
Annapurna A, Ansari MA, Manjunath PM. Partial role of multiple pathways in infarct size limiting effect of quercetin and rutin against cerebral ischemia-reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2013;17:491–500.
Scholz RW, Reddy PV, Wynn MK, Graham KS, Liken AD, Gumpricht E, et al. Glutathione dependent factors and inhibition of rat liver microsomal lipid peroxidation. Free Radic Biol Med. 1997;23:815–28.
Rajesh MG, Latha MS. Preliminary evaluation of antihepatotoxic activity of Kamilari, a poly herbal formulation. J Ethnopharmacol. 2004;91:99–104.
Braissant O, McLin VA, Cudalbu C. Ammonia toxicity to the brain. J Inherit Metab Dis. 2013;36:595–612.
Rao KV, Jayakumar AR, Tong X, Alvarez VM, Norenberg MD. Marked potentiation of cell swelling by cytokines in ammonia-sensitized cultured astrocytes. J Neuroinflam. 2010;7:66.
Acknowledgments
This research work was supported by the Indian Council of Medical Research ICMR45/3/2013-Bio/BMS dated 25.11.2013, New Delhi, India, in the form of Senior Research Fellowship to S. Kanimozhi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kanimozhi, S., Bhavani, P. & Subramanian, P. Influence of the Flavonoid, Quercetin on Antioxidant Status, Lipid Peroxidation and Histopathological Changes in Hyperammonemic Rats. Ind J Clin Biochem 32, 275–284 (2017). https://doi.org/10.1007/s12291-016-0603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0603-8