Abstract
Background
We investigated the usefulness of the minimum ADC value of primary breast lesions for predicting axillary lymph node (LN) status in luminal A-like breast cancers with clinically negative nodes in comparison with the mean ADC.
Methods
Forty-four luminal A-like breast cancers without axillary LN metastasis at preoperative clinical evaluation, surgically resected with sentinel LN biopsy, were retrospectively studied. Mean and minimum ADC values of each lesion were measured and statistically compared between LN positive (n = 12) and LN negative (n = 32) groups. An ROC curve was drawn to determine the best cutoff value to differentiate LN status. Correlations between mean and minimum ADC values and the number of metastatic axillary LNs were investigated.
Results
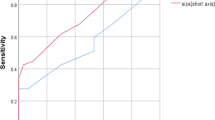
Mean and minimum ADC values of breast lesions with positive LN were significantly lower than those with negative LN (mean 839.9 ± 110.9 vs. 1022.2 ± 250.0 × 10− 6 mm2/s, p = 0.027, minimum 696.7 ± 128.0 vs. 925.0 ± 257.6 × 10− 6 mm2/s, p = 0.004). The sensitivity and NPV using the best cutoff value from ROC using both mean and minimum ADC were 100%. AUC of the minimum ADC (0.784) was higher than that of the mean ADC (0.719). Statistically significant negative correlations were observed between both mean and minimum ADCs and number of positive LNs, with stronger correlation to minimum ADC than mean ADC.
Conclusions
The minimum ADC value of primary breast lesions predicts axillary LN metastasis in luminal A-like breast cancer with clinically negative nodes, with high sensitivity and high NPV.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- LN:
-
Lymph node
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive value
- PgR:
-
Progesterone receptor
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
References
Curigliano G, Burstein HJ, Winner PE, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700–12.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551–7.
Chen X, Cong Y, Pan L, Jiang Y, Meng Q, Sun L, et al. Luminal (Her2 negative) prognostic index and survival of breast cancer patients. Cancer Epidemiol. 2014;38:286–90.
Yamashita H, Ogiya A, Shien T, Horimoto Y, Masuda N, Inao T, et al. Clinicopathological factors predicting early and late distant recurrence in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2016;23:830–43.
Wildiers H, Van Calster B, van de Poll-Franse LV, Hendrickx W, Roislien J, Smeets A, et al. Relationship between age and axillary lymph node involvement in women with breast cancer. J Clin Oncol. 2009;27:2931–7.
Rivadeneira DE, Simmons RM, Christos PJ, Hanna K, Daly JM, Osborne MP. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191:1–6 (discussion 8).
Yip CH, Taib NA, Tan GH, Ng KL, Yoong BK, Choo WY. Predictors of axillary lymph node metastases in breast cancer: is there a role for minimal axillary surgery? World J Surg. 2009;33:54–7.
Lyman GH, Somerfield MR, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: 2016 American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract. 2017;13:196–8.
Youk JH, Son EJ, Chung J, Kim JA, Kim EK. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol. 2012;22:1724–34.
Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging. 2009;30:615–20.
Martincich L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol. 2012;22:1519–28.
Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010;23:619–23.
Guvenc I, Akay S, Ince S, Yildiz R, Kilbas Z, Oysul FG, et al. Apparent diffusion coefficient value in invasive ductal carcinoma at 3.0 T: is it correlated with prognostic factors? Br J Radiol. 2016;89:20150614.
Kim JY, Seo HB, Park S, Moon JI, Lee JW, Lee NK, et al. Early-stage invasive ductal carcinoma: association of tumor apparent diffusion coefficient values with axillary lymph node metastasis. Eur J Radiol. 2015;84:2137–43.
Hirano M, Satake H, Ishigaki S, Ikeda M, Kawai H, Naganawa S. Diffusion-weighted imaging of breast masses: comparison of diagnostic performance using various apparent diffusion coefficient parameters. AJR Am J Roentgenol. 2012;198:717–22.
Mori N, Ota H, Mugikura S, Takasawa C, Tominaga J, Ishida T, et al. Detection of invasive components in cases of breast ductal carcinoma in situ on biopsy by using apparent diffusion coefficient MR parameters. Eur Radiol. 2013;23:2705–12.
Kato F, Kudo K, Yamashita H, Wang J, Hosoda M, Hatanaka KC, et al. Differences in morphological features and minimum apparent diffusion coefficient values among breast cancer subtypes using 3-tesla MRI. Eur J Radiol. 2016;85:96–102.
Brierley JD. Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Oxford: Wiley; 2016.
Sui WF, Chen X, Peng ZK, Ye J, Wu JT. The diagnosis of metastatic axillary lymph nodes of breast cancer by diffusion weighted imaging: a meta-analysis and systematic review. World J Surg Oncol. 2016;14:155.
Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55:736–42.
Inoue C, Fujii S, Kaneda S, Fukunaga T, Kaminou T, Kigawa J, et al. Apparent diffusion coefficient (ADC) measurement in endometrial carcinoma: effect of region of interest methods on ADC values. J Magn Reson Imaging. 2014;40:157–61.
Hida T, Nishie A, Asayama Y, Ishigami K, Ushijima Y, Takayama Y, et al. Apparent diffusion coefficient characteristics of various adrenal tumors. Magn Reson Med Sci. 2014;13:183–9.
Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–8.
Rosenkrantz AB, Sigmund EE, Winnick A, Niver BE, Spieler B, Morgan GR, et al. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: preliminary experience in fresh liver explants. Magn Reson Imaging. 2012;30:1534–40.
Tashireva LA, Denisov EV, Gerashchenko TS, Pautova DN, Buldakov MA, Zavyalova MV, et al. Intratumoral heterogeneity of macrophages and fibroblasts in breast cancer is associated with the morphological diversity of tumor cells and contributes to lymph node metastasis. Immunobiology. 2017;222:631–40.
Matsuoka A, Minato M, Harada M, Kubo H, Bandou Y, Tangoku A, et al. Comparison of 3.0-and 1.5-tesla diffusion-weighted imaging in the visibility of breast cancer. Radiat Med. 2008;26:15–20.
Latifoltojar A, Dikaios N, Ridout A, Moore C, Illing R, Kirkham A, et al. Evolution of multi-parametric MRI quantitative parameters following transrectal ultrasound-guided biopsy of the prostate. Prostate Cancer Prostatic Dis. 2015;18:343–51.
Acknowledgements
This study was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant numbers 26860962 and 17K10389) and supported by Global Station for Quantum Medical Science and Engineering, a project of Global Institution for Collaborative Research and Education at Hokkaido University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Kudo received research grants from Hitachi Ltd and Philips Medical systems, as well as lecture fees from Hitachi Ltd, GE Healthcare, Siemens Healthcare, and Toshiba Medical Systems. The other authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kato, F., Kudo, K., Yamashita, H. et al. Predicting metastasis in clinically negative axillary lymph nodes with minimum apparent diffusion coefficient value in luminal A-like breast cancer. Breast Cancer 26, 628–636 (2019). https://doi.org/10.1007/s12282-019-00969-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-019-00969-0