Abstract
Background
Cancer results from a series of molecular changes that alter the normal function of cells. Breast cancer is the second leading cause of cancer death in women. To develop novel anticancer agents, new series of chromen derivatives were synthesized and evaluated for their cytotoxic activity against human breast cancer cell lines.
Method
The growth inhibitory activities of synthesized hexahydrobenzo chromen-4-one were screened against six human cancer cell lines using an in vitro cell culture system (MTT assay). Fluorochrome staining (acridine orange/ethidium bromide double staining) and DNA fragmentation by the diphenylamine method were used to investigate the effects of most potent compounds on the process of apoptosis in breast cancer cell lines. To determine the mechanism of apoptosis, ROS and NOX production in treated breast cancer cells with compounds was evaluated.
Results
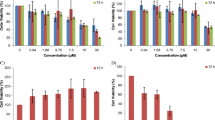
The cytotoxicity data of tested compounds demonstrate these compounds had varying degree of toxicity. Compound 7h was the most potent compound with IC50 = 1.8 ± 0.6 µg/mL against T-47D cell line. Analyses of the compounds treated (MCF-7, MDA-MB-231, and T-47D) cells by acridine orange/ethidium bromide double staining and DNA fragmentation by the diphenylamine method showed that the synthetic compounds induce apoptosis in the cells. A significant increase in ROS production was observed in T-47D cells treated with IC50 value of compound 7g. Incubation with IC50 value of synthetic compounds increased the NOX production in cell lines, especially T-47D cells.
Conclusion
Our results show that most compounds have a significant anti-proliferative activity against six human cancer cell lines. The observations confirm that chromen derivatives have induced the cell death through apoptosis.







Similar content being viewed by others
References
Lago JH, Ramos CS, Casanova DC, Morandim Ade A, Bergamo DC, Cavalheiro AJ, et al. Benzoic acid derivatives from Piper species and their fungitoxic activity against Cladosporium cladosporioides and C. sphaerospermum. J Nat Prod. 2004;67:1783–8.
Baldoqui DC, Kato MJ, Cavalheiro AJ, Bolzani VS, Young MC, Furlan M. A chromene and prenylated benzoic acid from Piper aduncum. Phytochemistry. 1999;51:899–902.
Liu XH, Li J, Wu FR, Song BA, Bhadury PS, Shi L. Novel 3-(2-(3-methyl-5-substituted-phenyl-4, 5-dihydropyrazol-1-yl)-2-oxoethoxy)-2-subst ituted-phenyl-4H-chromen-4-one: synthesis and anticancer activity, medicinal chemistry. Med Chem. 2011;7:605–10.
Aiello S, Wells G, Stone EL, Kadri H, Bazzi R, Bell DR, et al. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648). J Med Chem. 2008;51:5135–9.
Dey SK, Bose D, Hazra A, Naskar S, Nandy A, Munda RN, et al. Cytotoxic activity and apoptosis-inducing potential of di-spiropyrrolidino and di-spiropyrrolizidino oxindole andrographolide derivatives. PLoS One. 2013;8(3):e58055.
Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–63.
Sinha K, Das J, Pal PB, et al. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87(7):1157–80.
Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356(2–3):295–8.
Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16(6):1834–44.
Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12(5–6):311–20.
Yasuda H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: Nitric oxide donor as a therapeutic enhancer. Nitric Oxide. 2008;19(2):205–16.
Ostrakhovitch EA, Cherian MG. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis. 2005;10:111–21.
Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–68.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Baskić D, Popović S, Ristić P, Arsenijević NN. Analysis of cyclohexamide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32.
Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci U S A. 1999;96(14):8271–6.
LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31.
Feldman PL, Griffith OW, Hong H, Stuehr DJ. Irreversible inactivation of macrophage and brain nitric oxide synthase by L-NG-methylarginine requires NADPH-dependent hydroxylation. J Med Chem. 1993;36:491–6.
Gharavi N, El-Kadi AO. Measurement of nitric oxide in murine Hepatoma Hepa1c1c7 cells by reversed phase HPLC with fluorescence detection. J Pharm Pharmaceut Sci. 2003;6(2):302–7.
Griess P. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt “Ueber einige azoverbindungen”. EurJIC. 1879;12:426–8.
Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Mol Divers Preserv Int. 2003;3(8):276–84.
Natarajan N, Shambaugh GE, Elseth KM, Haines GK, Radosevich JA. Adaptation of the diphenylamine (DPA) assay to a 96-well plate tissue culture format and comparison with the MTT assay. Biotechniques. 1994;17:166–71.
Antosiewicz J, Ziolkowski W, Kar S, Powolny AA, Singh SV. Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 2008;74(13):1570–9.
Widlak P, Garrard WT. Discovery, regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J Cell Biochem. 2005;94:1078–87.
Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PRM. Apoptotic mechanisms in T-47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87(8):909–17.
Liu X, Zou H, Widlak P, Garrard W, Wang X. Activation of the apoptotic endonuclease DFF40 (Caspase activated Dnase or Nuclease). Oligomerisation and direct interaction with histone H1. J Biol Chem. 1999;274:13836–40.
Kheirollahi A, Pordeli M, Safavi M, Mashkouri S, Naimi-Jamal MR, Ardestani SK. Cytotoxic and apoptotic effects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn-Schmiedeberg’s Arch Pharmacol. 2014;387(12):1199–208.
Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300.
Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18(4):775–94.
Marchi S, Giorgi C, Suski JM, Agnoletto Chiara, Bononi Angela, Bonora Massimo, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635.
Oh S, Xiaofei E, Ni D, Pirooz SD, Lee JY, Lee D, et al. Down regulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011;18(3):452–64.
Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54(8):2095–7.
Jacobson Michael D, Raff Martin C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374(6525):814–6.
Amstad PA, Liu H, Ichimiya M, Berezesky IK, Trump BF, Buhimschi IA, et al. BCL-2 is involved in preventing oxidant-induced cell death and in decreasing oxygen radical production. Redox Rep. 2001;6(6):351–62.
Liu Bin, Chen Yumin. Daret K Clair. ROS and p53: versatile partnership. Free Radic Biol Med. 2008;44(8):1529–35.
Synder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS ONE. 2009;4(9):e7059.
Chen YK, Hsue SS, Lin LM. Correlation between inducible nitric oxide synthase and p53 expression for DMBA-induced hamster buccal-pouch carcinomas. Oral Dis. 2003;9(5):227–34.
Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front Physiol. 2013;4:144.
Messmer UK, Ankarcrona M, Nicotera P. Brune B.P53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355:23–6.
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Natl Acad Sci USA. 1996;93(6):2442–7.
Acknowledgments
This research has been supported by a grant from Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
About this article
Cite this article
Pordeli, M., Nakhjiri, M., Safavi, M. et al. Anticancer effects of synthetic hexahydrobenzo [g]chromen-4-one derivatives on human breast cancer cell lines. Breast Cancer 24, 299–311 (2017). https://doi.org/10.1007/s12282-016-0704-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0704-5