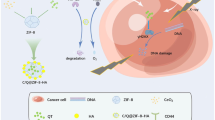
Abstract
Due to their complementary activity, the use of DNA damage repair (DDR) inhibitors during radiotherapy (RT) has yielded promising results. Unfortunately, this approach is often hindered by toxicity and poor in vivo stability of the DDR inhibitors. Nanoscale metal-organic frameworks (nMOFs) represent an emerging class of crystalline materials which exhibit advantageous properties over traditional nanomaterials and demonstrate great potential in oncology. Herein, a unique, synergistic treatment strategy for enhancing the therapeutic efficacy of RT via nMOF-mediated drug delivery and RT enhancement was evaluated. A nMOF containing the high-Z element Hf and the ligand 1,4-dicarboxybenzene (Hf-BDC) was synthesized and loaded with the two DDR inhibitor drugs, talazoparib and buparlisib (TB@Hf-BDC-PEG). TB@Hf-BDC-PEG augmented RT by increasing reactive oxygen species generation and thus DNA damage of which repair was inhibited thereafter. Synergistic enhancement was demonstrated in vivo where the combination of concurrent radiation with intravenous TB@Hf-BDC-PEG administration resulted in improved tumor control and increased apoptosis. Importantly, no apparent toxicity was observed with nMOF treatment, supporting its potential as an attractive candidate over traditional nanomaterials. This work provides the first report of nMOFs employed to enhance the therapeutic response to RT through DDR inhibitor delivery and physical radiation dose enhancement.

Similar content being viewed by others
References
Schaue, D.; McBride, W. H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol.2015, 12, 527–540.
Chatterjee, D. K.; Wolfe, T.; Lee, J.; Brown, A. P.; Singh, P. K.; Bhattarai, S. R.; Diagaradjane, P.; Krishnan, S. Convergence of nanotechnology with radiation therapy-insights and implications for clinical translation. Transl. Cancer Res.2013, 2, 256–268.
He, C. B.; Liu, D. M.; Lin, W. B. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev.2015, 115, 11079–11108.
Steel, G. G.; Peckham, M. J. Exploitable mechanisms in combined radiotherapy-chemotherapy: The concept of additivity. Int. J. Radiat. Oncol. Biol. Phys.1979, 5, 85–91.
Hartshorn, C. M.; Bradbury, M. S.; Lanza, G. M.; Nel, A. E.; Rao, J. H.; Wang, A. Z.; Wiesner, U. B.; Yang, L.; Grodzinski, P. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano2018, 12, 24–43.
Furukawa, H.; Cordova, K. E.; O’Keeffe, M.; Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science2013, 341, 1230444.
Wu, M. X.; Yang, Y. W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater.2017, 29, 1606134.
Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater.2018, 30, 1707365.
Liu, J. J.; Yang, Y.; Zhu, W. W.; Yi, X.; Dong, Z. L.; Xu, X. N.; Chen, M. W.; Yang, K.; Lu, G.; Jiang, L. X. et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials2016, 97, 1–9.
Chen, D. Q.; Yang, D. Z.; Dougherty, C. A.; Lu, W. F.; Wu, H. W.; He, X. R.; Cai, T.; van Dort, M. E.; Ross, B. D.; Hong, H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano2017, 11, 4315–4327.
Lu, K. D.; Aung, T.; Guo, N. N.; Weichselbaum, R.; Lin, W. B. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater.2018, 30, 1707634.
Lan, G. X.; Ni, K. Y.; Veroneau, S. S.; Song, Y.; Lin, W. B. Nanoscale metal-organic layers for radiotherapy-radiodynamic therapy. J. Am. Chem. Soc.2018, 140, 16971–16975.
Lu, K. D.; He, C. B.; Guo, N. N.; Chan, C.; Ni, K. Y.; Lan, G. X.; Tang, H. D.; Pelizzari, C.; Fu, Y. X.; Spiotto, M. T. et al. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng.2018, 2, 600–610.
Ni, K. Y.; Lan, G. X.; Veroneau, S. S.; Duan, X. P.; Song, Y.; Lin, W. B. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat. Commun.2018, 9, 4321.
Her, S.; Jaffray, D. A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Del. Rev.2017, 109, 84–101.
Lázaro, I. A.; Lázaro, S. A.; Forgan, R. S. Enhancing anticancer cytotoxicity through bimodal drug delivery from ultrasmall Zr MOF nanoparticles. Chem. Commun.2018, 54, 2792–2795.
Seiwert, T. Y.; Salama, J. K.; Vokes, E. E. The concurrent chemoradiation paradigm-general principles. Nat. Clin. Pract. Oncol.2007, 4, 86–100.
Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci.2014, 105, 370–388.
Dréan, A.; Lord, C. J.; Ashworth, A. PARP inhibitor combination therapy. Crit. Rev. Oncol. Hematol.2016, 108, 73–85.
Jang, N. Y.; Kim, D. H.; Cho, B. J.; Choi, E. J.; Lee, J. S.; Wu, H. G.; Chie, E. K.; Kim, I. A. Radiosensitization with combined use of olaparib and PI-103 in triple-negative breast cancer. BMC Cancer2015, 15, 89.
Philip, C. A.; Laskov, I.; Beauchamp, M. C.; Marques, M.; Amin, O.; Bitharas, J.; Kessous, R.; Kogan, L.; Baloch, T.; Gotlieb, W. H. et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer2017, 17, 638.
Yi, Y. W.; Park, J. S.; Kwak, S. J.; Seong, Y. S. Co-treatment with BEZ235 enhances sensitivity of BRCA1-negative breast cancer cells to olaparib. Anticancer Res.2015, 35, 3829–3838.
Wang, D.; Li, C.; Zhang, Y.; Wang, M.; Jiang, N.; Xiang, L.; Li, T.; Roberts, T. M.; Zhao, J. J.; Cheng, H. et al. Combined Inhibition of PI3K and PARP is effective in the treatment of ovarian cancer cells with wild-type PIK3CA genes. Gynecol. Oncol.2016, 142, 548–556.
Juvekar, A.; Burga, L. N.; Hu, H.; Lunsford, E. P.; Ibrahim, Y. H.; Balmañà, J.; Rajendran, A.; Papa, A.; Spencer, K.; Lyssiotis, C. A. et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov.2012, 2, 1048–1063.
Ibrahim, Y. H.; García-García, C.; Serra, V.; He, L.; Torres-Lockhart, K.; Prat, A.; Anton, P.; Cozar, P.; Guzmán, M.; Grueso, J. et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov.2012, 2, 1036–1047.
Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B2015, 5, 442–453.
DuRoss, A. N.; Neufeld, M. J.; Landry, M. R.; Rosch, J. G.; Eaton, C. T.; Sahay, G.; Thomas, C. R. Jr.; Sun, C. Micellar formulation of talazoparib and buparlisib for enhanced DNA damage in breast cancer chemoradiotherapy. ACS Appl. Mater. Interfaces2019, 11, 12342–12356.
Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal-organic frameworks: From nano to single crystals. Chem.–Eur. J.2011, 17, 6643–6651.
Morris, W.; Wang, S. Z.; Cho, D.; Auyeung, E.; Li, P.; Farha, O. K.; Mirkin, C. A. Role of modulators in controlling the colloidal stability and polydispersity of the UiO-66 metal-organic framework. ACS Appl. Mater. Interfaces2017, 9, 33413–33418.
He, T.; Xu, X. B.; Ni, B.; Wang, H. Q.; Long, Y.; Hu, W. P.; Wang, X. Fast and scalable synthesis of uniform zirconium-, hafnium-based metal-organic framework nanocrystals. Nanoscale2017, 9, 19209–19215.
Cavka, J. H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K. P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc.2008, 130, 13850–13851.
deKrafft, K. E.; Boyle, W. S.; Burk, L. M.; Zhou, O. Z.; Lin, W. B. Zr- and Hf-based nanoscale metal-organic frameworks as contrast agents for computed tomography. J. Mater. Chem.2012, 22, 18139–18144.
Che, J.; Okeke, C. I.; Hu, Z. B.; Xu, J. DSPE-PEG: A distinctive component in drug delivery system. Curr. Pharm. Des.2015, 21, 1598–1605.
Strojan, K.; Leonardi, A.; Bregar, V. B.; Križaj, I.; Svete, J.; Pavlin, M. Dispersion of nanoparticles in different media importantly determines the composition of their protein corona. PLoS One2017, 12, e0169552.
Zhu, X. Y.; Gu, J. L.; Wang, Y.; Li, B.; Li, Y. S.; Zhao, W. R.; Shi, J. L. Inherent anchorages in UiO-66 nanoparticles for efficient capture of alendronate and its mediated release. Chem. Commun.2014, 50, 8779–8782.
Rojas, S.; Colinet, I.; Cunha, D.; Hidalgo, T.; Salles, F.; Serre, C.; Guillou, N.; Horcajada, P. Toward understanding drug incorporation and delivery from biocompatible metal-organic frameworks in view of cutaneous administration. ACS Omega2018, 3, 2994–3003.
Cunha, D.; Gaudin, C.; Colinet, I.; Horcajada, P.; Maurin, G.; Serre, C. Rationalization of the entrapping of bioactive molecules into a series of functionalized porous zirconium terephthalate MOFs. J. Mater. Chem. B2013, 1, 1101–1108.
Cunha, D.; Ben Yahia, M.; Hall, S.; Miller, S. R.; Chevreau, H.; Elkaim, E.; Maurin, G.; Horcajada, P.; Serre, C. Rationale of drug encapsulation and release from biocompatible porous metal-organic frameworks. Chem. Mater.2013, 25, 2767–2776.
Lan, G. X.; Ni, K. Y.; Lin, W. B. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord. Chem. Rev.2019, 379, 65–81.
Chen, H. M.; Wang, G. D.; Chuang, Y. J.; Zhen, Z. P.; Chen, X. Y.; Biddinger, P.; Hao, Z. L.; Liu, F.; Shen, B. Z.; Pan, Z. W. et al. Nanoscintillator-mediated X-ray inducible photodynamic therapy for in vivo cancer treatment. Nano Lett.2015, 15, 2249–2256.
Chou, T. C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res.2010, 70, 440–446.
Albert, J. M.; Cao, C.; Kim, K. W.; Willey, C. D.; Geng, L.; Xiao, D. K.; Wang, H.; Sandler, A.; Johnson, D. H.; Colevas, A. D. et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin. Cancer Res.2007, 13, 3033–3042.
Banáth, J. P.; Klokov, D.; MacPhail, S. H.; Banuelos, C. A.; Olive, P. L. Residual γH2AX foci as an indication of lethal DNA lesions. BMC Cancer2010, 10, 4.
Banáth, J. P.; MacPhail, S. H.; Olive, P. L. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res.2004, 64, 7144–7149.
Klokov, D.; MacPhail, S. M.; Banáth, J. P.; Byrne, J. P.; Olive, P. L. Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother. Oncol.2006, 80, 223–229.
Subiel, A.; Ashmore, R.; Schettino, G. Standards and methodologies for characterizing radiobiological impact of high-Z nanoparticles. Theranostics2016, 6, 1651–1671.
Zheng, X. H.; Wang, L.; Liu, M.; Lei, P. P.; Liu, F.; Xie, Z. G. Nanoscale mixed-component metal-organic frameworks with photosensitizer spatial-arrangement-dependent photochemistry for multimodal-imaging-guided photothermal therapy. Chem. Mater.2018, 30, 6867–6876.
Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J. F.; Heurtaux, D.; Clayette, P.; Kreuz, C. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater.2010, 9, 172–178.
Baati, T.; Njim, L.; Neffati, F.; Kerkeni, A.; Bouttemi, M.; Gref, R.; Najjar, M. F.; Zakhama, A.; Couvreur, P.; Serre, C. et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal-organic frameworks. Chem. Sci.2013, 4, 1597–1607.
Hanahan, D.; Weinberg, R. A. Hallmarks of cancer: The next generation. Cell2011, 144, 646–674.
Knijnenburg, T. A.; Wang, L. H.; Zimmermann, M. T.; Chambwe, N.; Gao, G. F.; Cherniack, A. D.; Fan, H. H.; Shen, H.; Way, G. P.; Greene, C. S. et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep.2018, 23, 239–254.e6.
Pilié, P. G.; Tang, C.; Mills, G. B.; Yap, T. A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol.2019, 16, 81–104.
Tangutoori, S.; Baldwin, P.; Sridhar, S. PARP inhibitors: A new era of targeted therapy. Maturitas2015, 81, 5–9.
Galon, J.; Laé, M.; Thariat, J. O.; Carrere, S.; Papai, Z.; Delannes, M.; Sargos, P.; Rochaix, P.; Mangel, L. C.; Sapi, Z. et al. Hafnium oxide nanoparticle activated by radiation therapy generates an anti-tumor immune response. Int. J. Radit. Oncol.2018, 102, S204–S205.
Maggiorella, L.; Barouch, G.; Devaux, C.; Pottier, A.; Deutsch, E.; Bourhis, J.; Borghi, E.; Levy, L. Nanoscale radiotherapy with hafnium oxide nano-particles. Future Oncol.2012, 8, 1167–1181.
Ni, K. Y.; Lan, G. X.; Chan, C.; Quigley, B.; Lu, K. D.; Aung, T.; Guo, N. N.; La Riviere, P.; Weichselbaum, R. R.; Lin, W. B. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun.2018, 9, 2351.
Sadeghi-Naini, A.; Papanicolau, N.; Falou, O.; Zubovits, J.; Dent, R.; Verma, S.; Trudeau, M.; Boileau, J. F.; Spayne, J.; Iradji, S. et al. Quantitative ultrasound evaluation of tumor cell death response in locally advanced breast cancer patients receiving chemotherapy. Clin. Cancer Res.2013, 19, 2163–2174.
Lee, S. Y.; Ju, M. K.; Jeon, H. M.; Jeong, E. K.; Lee, Y. J.; Kim, C. H.; Park, H. G.; Han, S. I.; Kang, H. S. Regulation of tumor progression by programmed necrosis. Oxid. Med. Cell. Longev.2018, 2018, 3537471.
Hirayama, R.; Ito, A.; Tomita, M.; Tsukada, T.; Yatagai, F.; Noguchi, M.; Matsumoto, Y.; Kase, Y.; Ando, K.; Okayasu, R. et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat. Res.2009, 171, 212–218.
Koontz, B. F.; Verhaegen, F.; De Ruysscher, D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol.2017, 90, 20160441.
Acknowledgements
This work was supported by the National Institutes of Health NIGMS as a Maximizing Investigators’ Research Award, 1R35GM119839-01 and Oregon State University College of Pharmacy Start-up Funds. We thank the Histology Core and the Advanced Light Microscopy Core at Oregon Health and Science University for tissue analysis support. Additionally, we thank the Center for Electron Microscopy and Nanofabrication at Portland State University for electron microscopy support, and the Trace Element Analysis Laboratory at Portland State University for ICPMS support. Lastly, the authors thank Colin Eaton and Kyle Holmes for weighing organs, homogenizing tissue and data entry.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Neufeld, M.J., DuRoss, A.N., Landry, M.R. et al. Co-delivery of PARP and PI3K inhibitors by nanoscale metal–organic frameworks for enhanced tumor chemoradiation. Nano Res. 12, 3003–3017 (2019). https://doi.org/10.1007/s12274-019-2544-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2544-z